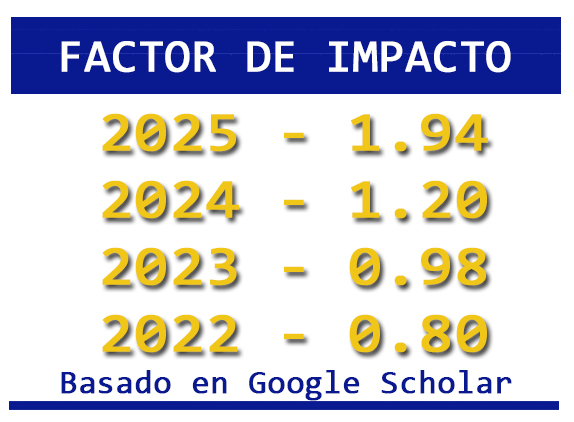
Harina de larva de mosca soldado negro y de organismos unicelulares como alternativas proteicas para alimentos acuícolas
DOI:
https://doi.org/10.36790/epistemus.v17i34.280Palabras clave:
Ingredientes alternativos, Acuacultura, Reemplazo, Harina de pescadoResumen
La búsqueda de ingredientes alternativos a la harina de pescado para su inclusión en alimento balanceado utilizado para el cultivo de organismos acuáticos es una tarea importante para la sustentabilidad de la acuacultura, una industria que satisface la demanda de alimentos acuáticos para el consumo humano. La harina de larva de mosca soldado negro y de organismos unicelulares como bacterias y levaduras, presentan contenidos de proteína relativamente altos, perfiles de amino ácidos favorables y se producen utilizando subproductos o residuos orgánicos de origen animal o vegetal, por lo que su producción es sustentable y de bajo costo, en comparación con la harina de pescado. El presente artículo de divulgación tiene por objeto dar a conocer las características más relevantes de estos insumos alternativos, así como mostrar algunos de los ejemplos más significativos de su utilización en alimentos balanceados para el cultivo de especies de importancia comercial.
Descargas
Citas
National Research Council (NRC), “Nutrient Requirements of Fish and Shrimp,” Washington, D.C.: The National Academies Press, 2011, pp. 70–71, DOI: https://doi.org/10.17226/130.39
Food and Agriculture Organization of the United Nations (FAO), “The State of World Fisheries and Aquaculture”, Rome, 2020, pp. 1–244, DOI: https://doi.org/10.4060/ca9229en DOI: https://doi.org/10.4060/ca9229en
M. Marotti, D. Tome, and P. P. Mirand, “Converting nitrogen into protein-beyond 6.25 and Jones’ Factors,” Critical Reviews in Food Science and Nutrition, 2008, vol. 48, pp. 177–184. DOI: http://10.1080/10408390701279749 DOI: https://doi.org/10.1080/10408390701279749
National Research Council (NRC), “Nutrient Requirements of Swine,” Washington, D.C.: The National Academies Press, 1998, pp. 124-141, ISBN 9780309132640
M. Shumo, I. M. Osuga, F. M. Khamis, C. M. Tanga, K. K. Fiaboe, S. Subramanian, S. Ekesi, A. van Huis, and C. Borgemeister, “The nutritive value od black soldier fly larvae reared on common organic waste streams in Kenya,” Scientific Reports, 2019, vol. 9, pp. 1-13. DOI: https://doi.org/10.1038/s41598-019-46603-z DOI: https://doi.org/10.1038/s41598-019-46603-z
M. D. Erdman, W. G. Bergen, and C. Adinarayana Reddy, “Amino acid profiles and presumptive nutritional assestment of Single-Cell Protein from certain Lactobacilli,” Applied and Environmental Microbiology, 1977, vol. 33, pp. 901-905. 10.1128/aem.33.4.901-905.1977 DOI: https://doi.org/10.1128/aem.33.4.901-905.1977
K. B. Barragan-Fonseca, M. Dicke, and J. J. A. van Loon, “Nutritinal value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed – a review,” Journal of Insects as Food and Feed, 2017, vol. 3, pp. 105–120. DOI: https://doi.org/10.3920/JIFF2016.0055 DOI: https://doi.org/10.3920/JIFF2016.0055
R. Rachmawati, D. Buchori, P. Hidayat, S. Hem, and M. R. Fahmi, “Perkembangan dan kandungan nutrisi larva Hermetia illucens (Linnaeus)(Diptera: Stratiomyidae) pada bungkil kelapa sawit”. Jurnal Entomologi Indonesia, 2010, vol. 7, pp. 28–41. DOI: https://doi.org/10.5994/jei.7.1.28 DOI: https://doi.org/10.5994/jei.7.1.28
K. B. Barragan-Fonseca, M. Dicke, and J. J. A. van Loon, “Influence of larval density and dietary nutrient concentration on performance, body protein, and fat contents of black soldier fly larvae (Hermetia illucens),” Entomologia Experimentalis et Applicata, 2018, vol.166(9), pp. 761–770. DOI: https://doi.org/10.1111/eea.12716 DOI: https://doi.org/10.1111/eea.12716
S. Kroeckel, A. G. E. Harjes, I. Roth, H. Katz, S. Wuertz, A. Susenbeth, and C. Schulz, “When a turbot catches a fly: Evaluation of a pre-pupae meal of the Black Soldier Fly (Hermetia illucens) as fish meal substitute - Growth performance and chitin degradation in juvenile turbot (Psetta maxima),” Aquaculture, 2012, vol. 364–365, pp. 345–352. DOI: https://doi.org/10.1016/j.aquaculture.2012.08.041 DOI: https://doi.org/10.1016/j.aquaculture.2012.08.041
A. Benzertiha, B. Kierończyk, M. Rawski, Z. Mikołajczak, A. Urbański, L. Nogowski, and D. Józefiak, “Insect Fat in Animal Nutrition – A Review,” Annals of Animal Science, 2020, vol. 20, no. 4, pp. 1217–1240. DOI: https://doi.org/10.2478/aoas-2020-0076 DOI: https://doi.org/10.2478/aoas-2020-0076
K. M. Rana, M. A. Salam, and M. Ariful-Islam M., “Development of black soldier fly larvae production technique as an alternate fish feed,” International Journal of Research in Fisheries and Aquaculture, 2015, vol. 5, no. 1, pp. 41–47.
A. Gougbedji, P. Agbohessou, P. Laleye, F. Francis, and R. Caparros-Megido R., “Technical basis for the small-scale production of black soldier fly, Hermetia illucens (L. 1758), meal as fish feed in Benin. Journal of Agriculture and Food Research, 2021, vol. 4., pp. 100153, DOI: https://doi.org/10.1016/j.jafr.2021.100153 DOI: https://doi.org/10.1016/j.jafr.2021.100153
L. Bruni, R. Pastorelli, C. Viti, L. Gasco, and G. Parisi, “Characterisation of the intestinal microbial communities of rainbow trout (Onchorhynchus mykiss) fed with Hermetia ilucens (black soldier fly) partially defatted larva meal as partial dietary protein source,” Aquaculture, 2018, vol. 487, pp. 56–63, DOI: https://doi.org/10.1016/j.aquaculture.2018.01.006 DOI: https://doi.org/10.1016/j.aquaculture.2018.01.006
S. Li, H. Ji, B. Zhang, J. Zhou, and H. Yu, “Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp (Cyprinus carpio var. Jian): Growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological structure,” Aquaculture, 2017, vol. 477, pp. 62–70. DOI: https://doi.org/10.1016/j.aquaculture.2017.04.015 DOI: https://doi.org/10.1016/j.aquaculture.2017.04.015
C. Caimi, M. Renna, C. Luissiana, A. Bonaldo, M. Gariglio, M. Meneguz, S. Dabbou, A. Schiavone, F. Gai, A. Concetta-Elia, M. Prearo, and L.Gasco, “First insights on black soldier fly (Hermetia illucens L.) larva meal dietary administration in Siberia sturgeon (Acipneser baerii Brandt) juveniles,” Aquaculture, 2020, vol. 515, pp. 734539, DOI: https://doi.org/10.1016/j.aquaculture.2019.734539 DOI: https://doi.org/10.1016/j.aquaculture.2019.734539
K. Mohan, D. K. Rajan, T. Muralisankar, A. R. Ganesan, P. Satishkumar, and N. Revathi, “Use of black soldier fly (Hermetia illucens L.) larvae meal in aquafeeds for a sustainable aquaculture industry: A review of past and future needs,” Aquaculture, 2022, vol. 553, pp. 738095 DOI: https://doi.org/10.1016/j.aquaculture.2022.738095 DOI: https://doi.org/10.1016/j.aquaculture.2022.738095
P. J. Strong, S. Xie, and W. P. Clarke, “Methane as a resource: can the methanotrophs add value?,” Environmental Science & Technology, 2015, vol. 49, no. 7, pp. 4001–4018, DOI: https://doi.org/10.1021/es504242n DOI: https://doi.org/10.1021/es504242n
R. Rudravaram, A. Chandel, L. V. Rao, Y. Hui, and P. Ravindra, “Bio (Single Cell) protein: issues of production, toxins and commercialization status,” in Agricultural Wastes, Eds G. S. Ashworth and P. Azevedo, New York: Nova Science Publishers, Inc., 2009, pp. 129–153.
A. Ritala, S. Häkkinen, M. Toivari, and M. Wiebe, “Single Cell Protein–State-of-the-Art, Industrial Landscape and Patents 2001–2016,” Frontiers in Microbiology, 2017, vol., 8, pp. 1–18, DOI: https://doi.org./10.3389/fmicb.2017.02009 DOI: https://doi.org/10.3389/fmicb.2017.02009
Q. Zhang, H. Liang, M. Longshaw, J. Wang, X. Ge, J. Zhu, S. Li, and M. Ren, “Effects of replacing fishmeal with methanotroph (Methylococcus capsulatus, Bath) bacteria meal (FeedKind®) on growth and intestinal health status of juvenile largemouth bass (Micropterus salmoides),” Fish and Shellfish Immunology, 2022, vol. 122, pp. 298–305. DOI: https://doi.org/10.1016/j.fsi.2022.02.008 DOI: https://doi.org/10.1016/j.fsi.2022.02.008
M. K. H. Chama, H. Liang, D. Huang, X. De, M. Ren, L. Zhang, L. Wu, and J. Ke, “Methanotroph (Methylococcus capsulatus, Bath) as an alternative protein source for genetically improved farmed tilapia (GIFT: Oreochromis niloticus) and its effect on antioxidants and immune response,” Aquaculture Reports, 2021, vol. 21, pp. 100872. DOI: https://doi.org/10.1016/j.aqrep.2021.100872 DOI: https://doi.org/10.1016/j.aqrep.2021.100872
A. Zamani, M. Khajavi, M. H. Nazarpak, and E. Gisbert, “Evaluation of a Bacterial Single-Cell Protein in Compound Diets for Rainbow Trout (Oncorhynchus mykiss) Fry as an Alternative Protein Source,” Animals, 2020, vol.10 no. 9, pp. 1676, DOI: https://doi.org/10.3390/ani10091676 DOI: https://doi.org/10.3390/ani10091676
A. Hamidoghli, H. Yun, S. Won, S. Kim., N. Farris, and S. C. Bai, “Evaluation of a single‑cell protein as a dietary fish meal substitute for whiteleg shrimp Litopenaeus vannamei,” Fisheries Science, 2018, vol. 85, pp. 147–155, DOI: https://doi.org/10.1007/s12562-018-1275-5 DOI: https://doi.org/10.1007/s12562-018-1275-5
A. T. Nasseri, S. Rasoul-Amini, M. H. Morowvat, and Y. Ghasemi, “Single cell protein: production and process,” American Journal of Food Technology, 2011, vol. 6, pp. 103–116. DOI: https://doi.org/10.3389/fmicb.2017.02009 DOI: https://doi.org/10.3923/ajft.2011.103.116
J. O. Hansen, M. Hofossaeter, C. Sahlmann, R. Anestad, F. E. Reveco-Urzua, C. M. Press, L. T. Mydland, and M. Overland, “Effect of Candida utilis on growth and intestinal health of Atlantic salmon (Salmo salar) parr,” Aquaculture, 2019, vol. 511, pp. 734239, DOI: https://doi.org/10.1016/j.aquaculture.2019.734239 DOI: https://doi.org/10.1016/j.aquaculture.2019.734239
J. Guo, X. Qiu, X., G. Salze, D. A. Davis, “Use of high-protein brewer’s yeast products in practical diets for the Pacific white shrimp Litopenaeus vannamei,” Aquaculture Nutrition, 2019, vol. 25, pp. 680–690. DOI: https://doi.org/10.1111/anu.12889 DOI: https://doi.org/10.1111/anu.12889
J. Gamboa-Delgado, B. Fernandez-Diaz, M. G. Nieto-Lopez, L. E. Cruz-Suarez, “Nutritional contribution of torula yeast and fish meal to the growth of shrimp Litopenaeus vannamei as indicated by natural nitrogen stable isotopes,” Aquaculture, 2015, vol. 453, pp. 116–121, DOI: https://doi.org/10.1016/j.aquaculture.2015.11.026 DOI: https://doi.org/10.1016/j.aquaculture.2015.11.026
R. Yossa, A. M. Greiling, R. K. Basiita, M. E. Sakala, W. A. Baumgartner, A. Taylor, D. M. Gatlin III, “Replacing fishmeal with a single cell protein feedstuff in Nile tilapia Oreochromis niloticus diets,” Animal Feed Science and Technology, 2021, vol. 281, pp., 115089. DOI: https://doi.org/10.1016/j.anifeedsci.2021.115089 DOI: https://doi.org/10.1016/j.anifeedsci.2021.115089

Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2023 EPISTEMUS

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
La revista adquiere los derechos patrimoniales de los artículos sólo para difusión sin ningún fin de lucro, sin menoscabo de los propios derechos de autoría.
Los autores son los legítimos titulares de los derechos de propiedad intelectual de sus respectivos artículos, y en tal calidad, al enviar sus textos expresan su deseo de colaborar con la Revista Epistemus, editada semestralmente por la Universidad de Sonora.
Por lo anterior, de manera libre, voluntaria y a título gratuito, una vez aceptado el artículo para su publicación, ceden sus derechos a la Universidad de Sonora para que la Universidad de Sonora edite, publique, distribuya y ponga a disposición a través de intranets, internet o CD dicha obra, sin limitación alguna de forma o tiempo, siempre y cuando sea sin fines de lucro y con la obligación expresa de respetar y mencionar el crédito que corresponde a los autores en cualquier utilización que se haga del mismo.
Queda entendido que esta autorización no es una cesión o transmisión de alguno de sus derechos patrimoniales en favor de la mencionada institución. La UniSon le garantiza el derecho de reproducir la contribución por cualquier medio en el cual usted sea el autor, sujeto a que se otorgue el crédito correspondiente a la publicación original de la contribución en Epistemus.
Salvo indicación contraria, todos los contenidos de la edición electrónica se distribuyen bajo una licencia de uso y Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0) Puede consultar desde aquí la versión informativa y el texto legal de la licencia. Esta circunstancia ha de hacerse constar expresamente de esta forma cuando sea necesario.