The Pharmaceutical Potential of Snakes Venoms Sonora, Mexico
DOI:
https://doi.org/10.36790/epistemus.v16i33.226Keywords:
Snakes, Venoms, Pharmacology, Biology, BiotechnologyAbstract
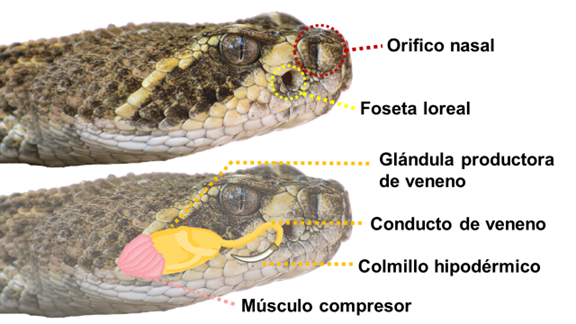
Venoms are complex biomolecules mixtures that are produced in specialized glands in several plants or animals. It has been reported that peptides and proteins are the venoms’ major components and are responsible for most of the clinical symptoms derived from a sting/bite. Additionally, snakes are probably the most representative venomous animals, due to cultural and medicinal reasons. Among these, rattlesnakes are highly feared, and in many cases, their venoms have been poorly studied. In the state of Sonora, Mexico, 12 species of rattlesnakes have been described, all considered as highly venomous since their bite requires medical attention. It has been reported that some of these species venoms’ components present antibacterial and anticancer activity, among many others. In the present work, we briefly describe how some of these venom components found in the venoms of Sonoran rattlesnakes, are of high pharmaceutical and biotechnological importance and should be paid attention.
Downloads
References
T. Tasoulis and G. Isbister, “A Review and Database of Snake Venom Proteomes,” Toxins, vol. 9, no. 9, p. 290, Sep. 2017, doi: 10.3390/toxins9090290. DOI: https://doi.org/10.3390/toxins9090290
B. Fry, Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery. Oxford University Press, 2015.
L. Fernández-Badillo, I. Zuria, J. J. Sigala-Rodríguez, G. Sanchez-Rojas, and G. Castaneda, “Revisión del conflicto humano-serpiente en México.pdf,” Animal Biodiversity and Conservation, vol. 44, pp. 153–174, May 2021, doi: 10.32800/abc.2021.44.0153. DOI: https://doi.org/10.32800/abc.2021.44.0153
“THE REPTILE DATABASE.” http://www.reptile-database.org/ (accessed Feb. 19, 2020).
J. C. Rorabaugh and J. A. Lemos-Espinal, A Field Guide to the Amphibians and Reptiles of Sonora, vol. 1. Rodeo, New Mexico: ECO Herpetological Publishing and Distribution, 2016.
J. A. Lemos-Espinal, G. R. Smith, and J. C. Rorabaugh, “A conservation checklist of the amphibians and reptiles of Sonora, Mexico, with updated species lists,” ZK, vol. 829, pp. 131–160, Mar. 2019, doi: 10.3897/zookeys.829.32146. DOI: https://doi.org/10.3897/zookeys.829.32146
“OMS - Mordeduras de serpientes venenosas.” https://www.who.int/es/news-room/fact-sheets/detail/snakebite-envenoming (accessed May 23, 2022).
“BoletínEpidemiológico Sistema Nacional de Vigilancia Epidemiológica Sistema Único de Información | Secretaría de Salud | Gobierno | gob.mx.” https://www.gob.mx/salud/documentos/boletinepidemiologico-sistema-nacional-de-vigilancia-epidemiologica-sistema-unico-de-informacion-231750 (accessed Jun. 11, 2020).
A. Alangode, K. Rajan, and B. G. Nair, “Snake antivenom: Challenges and alternate approaches,” Biochemical Pharmacology, vol. 181, p. 114135, Nov. 2020, doi: 10.1016/j.bcp.2020.114135. DOI: https://doi.org/10.1016/j.bcp.2020.114135
M. Sánchez et al., “Toxicological profile of medically relevant Crotalus species from Mexico and their neutralization by a Crotalus basiliscus/Bothrops asper antivenom,” Toxicon, vol. 179, pp. 92–100, May 2020, doi: 10.1016/j.toxicon.2020.03.006. DOI: https://doi.org/10.1016/j.toxicon.2020.03.006
A. Deshwal, P. Phan, J. Datta, R. Kannan, and S. K. Thallapuranam, “A Meta-Analysis of the Protein Components in Rattlesnake Venom,” Toxins, vol. 13, no. 6, Art. no. 6, Jun. 2021, doi: 10.3390/toxins13060372. DOI: https://doi.org/10.3390/toxins13060372
L. A. Calderon et al., “Antitumoral Activity of Snake Venom Proteins: New Trends in Cancer Therapy,” BioMed Research International, vol. 2014, pp. 1–19, 2014, doi: 10.1155/2014/203639. DOI: https://doi.org/10.1155/2014/203639
J. J. Calvete, E. Fasoli, L. Sanz, E. Boschetti, and P. G. Righetti, “Exploring the Venom Proteome of the Western Diamondback Rattlesnake, Crotalus atrox , via Snake Venomics and Combinatorial Peptide Ligand Library Approaches,” J. Proteome Res., vol. 8, no. 6, pp. 3055–3067, Jun. 2009, doi: 10.1021/pr900249q. DOI: https://doi.org/10.1021/pr900249q
Á. Segura et al., “Proteomic, toxicological and immunogenic characterization of Mexican west-coast rattlesnake (Crotalus basiliscus) venom and its immunological relatedness with the venom of Central American rattlesnake (Crotalus simus),” J Proteomics, vol. 158, pp. 62–72, Mar. 2017, doi: 10.1016/j.jprot.2017.02.015. DOI: https://doi.org/10.1016/j.jprot.2017.02.015
E. P. Hofmann et al., “Comparative venom-gland transcriptomics and venom proteomics of four Sidewinder Rattlesnake (Crotalus cerastes) lineages reveal little differential expression despite individual variation,” Sci Rep, vol. 8, no. 1, p. 15534, Dec. 2018, doi: 10.1038/s41598-018-33943-5. DOI: https://doi.org/10.1038/s41598-018-33943-5
R. M. Rautsaw et al., “Intraspecific sequence and gene expression variation contribute little to venom diversity in sidewinder rattlesnakes ( Crotalus cerastes),” Proc Biol Sci, vol. 286, no. 1906, p. 20190810, Jul. 2019, doi: 10.1098/rspb.2019.0810. DOI: https://doi.org/10.1098/rspb.2019.0810
M. Borja et al., “Morulustatin, A Disintegrin that Inhibits ADP-Induced Platelet Aggregation, Isolated from the Mexican Tamaulipan Rock Rattlesnake (Crotalus lepidus morulus),” Revista cientifica (Universidad del Zulia. Facultad de Ciencias Veterinarias. Division de Investigacion), vol. 26, no. 2, pp. 86–94, 2016.
“Intra-specific Variation in the Protein Composition and Proteolytic Activity of Venom of Crotalus lepidus morulus from the Northeast of Mexico.” https://bioone.org/journals/copeia/volume-2013/issue-4/OT-13-005/Intra-specific-Variation-in-the-Protein-Composition-and-Proteolytic-Activity/10.1643/OT-13-005.full (accessed May 09, 2022). DOI: https://doi.org/10.1643/OT-13-005
J. Jimenez-Canale et al., “Cytotoxic activity of Crotalus molossus molossus snake venom-loaded in chitosan nanoparticles against T-47D breast carcinoma cells,” Acta Biochimica Polonica, vol. 69, no. 1, Art. no. 1, Feb. 2022, doi: 10.18388/abp.2020_5975. DOI: https://doi.org/10.18388/abp.2020_5975
M. Borja et al., “Ontogenetic Change in the Venom of Mexican Black-Tailed Rattlesnakes (Crotalus molossus nigrescens),” Toxins, vol. 10, no. 12, p. 501, Dec. 2018, doi: 10.3390/toxins10120501. DOI: https://doi.org/10.3390/toxins10120501
K. K. Tan, S. G. Ler, J. Gunaratne, B. H. Bay, and G. Ponnampalam, “In vitro cytotoxicity of L-amino acid oxidase from the venom of Crotalus mitchellii pyrrhus,” Toxicon, vol. 139, pp. 20–30, Dec. 2017, doi: 10.1016/j.toxicon.2017.09.012. DOI: https://doi.org/10.1016/j.toxicon.2017.09.012
M. Borja et al., “Biological and Proteolytic Variation in the Venom of Crotalus scutulatus scutulatus from Mexico,” Toxins, vol. 10, no. 1, p. 35, Jan. 2018, doi: 10.3390/toxins10010035. DOI: https://doi.org/10.3390/toxins10010035
D. J. Massey et al., “Venom variability and envenoming severity outcomes of the Crotalus scutulatus scutulatus (Mojave rattlesnake) from Southern Arizona,” Journal of Proteomics, vol. 75, no. 9, pp. 2576–2587, May 2012, doi: 10.1016/j.jprot.2012.02.035. DOI: https://doi.org/10.1016/j.jprot.2012.02.035
J. J. Calvete et al., “Snake Venomics of Crotalus tigris: The Minimalist Toxin Arsenal of the Deadliest Neartic Rattlesnake Venom" J Proteome Res, vol. 11, no. 2, pp. 1382–1390, Feb. 2012, doi: 10.1021/pr201021d. DOI: https://doi.org/10.1021/pr201021d
C. M. Adade, S. F. Anne Cristine, O. C. Ana Lúcia, R. B. Zingali, and T. Souto-Padrón, “44. Leishmanicidal Effects of a Phospholipase A2 Isolated from Crotalus viridis viridis Snake Venom,” Toxicon, vol. 60, no. 2, p. 117, Aug. 2012, doi: 10.1016/j.toxicon.2012.04.045. DOI: https://doi.org/10.1016/j.toxicon.2012.04.045
C. M. Adade, B. L. Cons, P. A. Melo, and T. Souto-Padrón, “Effect of Crotalus viridis viridis snake venom on the ultrastructure and intracellular survival of Trypanosoma cruzi,” Parasitology, vol. 138, no. 1, pp. 46–58, Jan. 2011, doi: 10.1017/S0031182010000958. DOI: https://doi.org/10.1017/S0031182010000958
A. J. Saviola, A. J. Gandara, R. W. Bryson, and S. P. Mackessy, “Venom phenotypes of the Rock Rattlesnake (Crotalus lepidus) and the Ridge-nosed Rattlesnake (Crotalus willardi) from México and the United States,” Toxicon, vol. 138, pp. 119–129, Nov. 2017, doi: 10.1016/j.toxicon.2017.08.016. DOI: https://doi.org/10.1016/j.toxicon.2017.08.016
J. Dobson et al., “Rattling the border wall: Pathophysiological implications of functional and proteomic venom variation between Mexican and US subspecies of the desert rattlesnake Crotalus scutulatus,” Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, vol. 205, pp. 62–69, Feb. 2018, doi: 10.1016/j.cbpc.2017.10.008. DOI: https://doi.org/10.1016/j.cbpc.2017.10.008
E.-R. M. Redwan, “Animal-Derived Pharmaceutical Proteins,” Journal of Immunoassay and Immunochemistry, vol. 30, no. 3, pp. 262–290, Jun. 2009, doi: 10.1080/15321810903084400. DOI: https://doi.org/10.1080/15321810903084400
B. Lomonte, Y. Angulo, M. Sasa, and J. M. Gutierrez, “The Phospholipase A2 Homologues of Snake Venoms: Biological Activities and Their Possible Adaptive Roles,” 2009. https://www.ingentaconnect.com/content/ben/ppl/2009/00000016/00000008/art00003 (accessed Mar. 31, 2020).
J. M. Gutiérrez and A. Rucavado, “Snake venom metalloproteinases:Their role in the pathogenesis of local tissue damage,” Biochimie, vol. 82, no. 9, pp. 841–850, Sep. 2000, doi: 10.1016/S0300-9084(00)01163-9. DOI: https://doi.org/10.1016/S0300-9084(00)01163-9
J. Boldrini-França et al., “Beyond hemostasis: a snake venom serine protease with potassium channel blocking and potential antitumor activities,” Sci Rep, vol. 10, no. 1, p. 4476, Mar. 2020, doi: 10.1038/s41598-020-61258-x. DOI: https://doi.org/10.1038/s41598-020-61258-x
M. Shibuya et al., “Antimetastatic effect of defibrinogenation with batroxobin depends on the natural killer activity of host in mice,” J Cancer Res Clin Oncol, vol. 116, no. 2, pp. 168–172, Mar. 1990, doi: 10.1007/BF01612672. DOI: https://doi.org/10.1007/BF01612672
S.-M. Suhr and D.-S. Kim, “Identification of the Snake Venom Substance That Induces Apoptosis,” Biochemical and Biophysical Research Communications, vol. 224, no. 1, pp. 134–139, Jul. 1996, doi: 10.1006/bbrc.1996.0996. DOI: https://doi.org/10.1006/bbrc.1996.0996
L. Lavín de Juan, V. García Recio, P. Jiménez López, T. Girbés Juan, M. Cordoba-Diaz, and D. Cordoba-Diaz, “Pharmaceutical applications of lectins,” Journal of Drug Delivery Science and Technology, vol. 42, pp. 126–133, Dec. 2017, doi: 10.1016/j.jddst.2017.05.018. DOI: https://doi.org/10.1016/j.jddst.2017.05.018
S. Sarray, N. Srairi, J. Luis, J. Marvaldi, M. E. Ayeb, and N. Marrakchi, “Lebecetin, a C-Lectin Protein from the Venom of Macrovipera lebetina That Inhibits Platelet Aggregation and Adhesion of Cancerous Cells,” PHT, vol. 31, no. 3–6, pp. 173–176, 2001, doi: 10.1159/000048060. DOI: https://doi.org/10.1159/000048060
E. Rivas Mercado et al., “Disintegrins extracted from totonacan rattlesnake (Crotalus totonacus) venom and their anti-adhesive and anti-migration effects on MDA-MB-231 and HMEC-1 cells,” Toxicology in Vitro, vol. 65, p. 104809, Jun. 2020, doi: 10.1016/j.tiv.2020.104809. DOI: https://doi.org/10.1016/j.tiv.2020.104809
B. Akhtar, F. Muhammad, A. Sharif, and M. I. Anwar, “Mechanistic insights of snake venom disintegrins in cancer treatment,” European Journal of Pharmacology, vol. 899, p. 174022, May 2021, doi: 10.1016/j.ejphar.2021.174022. DOI: https://doi.org/10.1016/j.ejphar.2021.174022
K. J. McClellan and K. L. Goa, “Tirofiban,” Drugs, vol. 56, no. 6, pp. 1067–1080, Dec. 1998, doi: 10.2165/00003495-199856060-00017. DOI: https://doi.org/10.2165/00003495-199856060-00017
R. M. Scarborough, “Development of eptifibatide,” American Heart Journal, vol. 138, no. 6, pp. 1093–1104, Dec. 1999, doi: 10.1016/S0002-8703(99)70075-X. DOI: https://doi.org/10.1016/S0002-8703(99)70075-X
E. E. N. Castro et al., “Serpientes Venenosas en México: Una Revisión al Estudio de los Venenos, los Antivenenos y la Epidemiología," Revista Latinoamericana de Herpetología, vol. 3, no. 2, Art. no. 2, Nov. 2020, doi: 10.22201/fc.25942158e.2020.2.205. DOI: https://doi.org/10.22201/fc.25942158e.2020.2.205
T. Mohamed Abd El-Aziz, A. G. Soares, and J. D. Stockand, “Snake Venoms in Drug Discovery: Valuable Therapeutic Tools for Life Saving,” Toxins, vol. 11, no. 10, Art. no. 10, Oct. 2019, doi: 10.3390/toxins11100564. DOI: https://doi.org/10.3390/toxins11100564
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 EPISTEMUS

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The magazine acquires the patrimonial rights of the articles only for diffusion without any purpose of profit, without diminishing the own rights of authorship.
The authors are the legitimate owners of the intellectual property rights of their respective articles, and in such quality, by sending their texts they express their desire to collaborate with the Epistemus Magazine, published biannually by the University of Sonora.
Therefore, freely, voluntarily and free of charge, once accepted the article for publication, they give their rights to the University of Sonora for the University of Sonora to edit, publish, distribute and make available through intranets, Internet or CD said work, without any limitation of form or time, as long as it is non-profit and with the express obligation to respect and mention the credit that corresponds to the authors in any use that is made of it.
It is understood that this authorization is not an assignment or transmission of any of your economic rights in favor of the said institution. The University of Sonora guarantees the right to reproduce the contribution by any means in which you are the author, subject to the credit being granted corresponding to the original publication of the contribution in Epistemus.
Unless otherwise indicated, all the contents of the electronic edition are distributed under a license for use and Creative Commons — Attribution-NonCommercial-ShareAlike 4.0 International — (CC BY-NC-SA 4.0) You can consult here the informative version and the legal text of the license. This circumstance must be expressly stated in this way when necessary.
The names and email addresses entered in this journal will be used exclusively for the purposes established in it and will not be provided to third parties or for their use for other purposes.