Voltamperometric analysis of the reductive decomposition of chalcopyrite acetic acid-water system
DOI:
https://doi.org/10.36790/epistemus.v17i34.261Keywords:
chalcopyrite, acetic acid, reduction, kinetics, characterizationAbstract
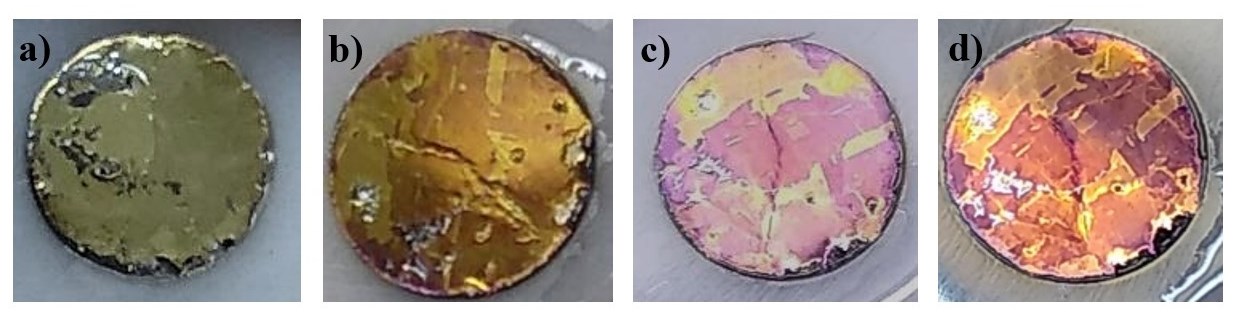
In this work, an electrochemical analysis of the reduction of chalcopyrite is presented using an aqueous medium in the presence of a weak acid such as acetic acid, in order to find the most favorable energetic conditions to dissolve the iron contained in the chalcopyrite and thereby reduce the passivation phenomenon. The investigation was carried out at the microelectrolysis level, analyzing different variables such as electrolyte concentration, electrolyte conductivity and applied potential on the chalcopyrite reduction kinetics. This study was complemented with a characterization of the surface of the reduced chalcopyrite using the FESEM technique and with a quantification of the iron that is released in the solution of the most important cathodic polarizations.
Downloads
References
J. C. Fuentes-Aceituno, G. T. Lapidus, and F. M. Doyle, “A kinetic study of the electro-assisted reduction of chalcopyrite,” Hydrometallurgy, vol. 92, no. 1–2, pp. 26–33, May 2008. DOI: 10.1016/j.hydromet.2008.02.002. DOI: https://doi.org/10.1016/j.hydromet.2008.02.002
W. G. Davenport, M. King, M. Schlesinger, and A. K. Biswas, Extractive metallurgy of copper, Fourth Edition. 2002.
F. Habashi and T. Toor, “Aqueous Oxidation of Chalcopyrite in Hydrochloric Acid,” Metallurgical Transactions B, pp. 49–56, Mar. 1979. DOI: 10.1007/BF02653971. DOI: https://doi.org/10.1007/BF02653971
Antonijevic, M.M., Jankovic, Z.D. and Dimitrijevic, M.D., Kinetics of Chalcopyrite Dissolution by Hydrogen Peroxide in Sulphuric Acid. Hydrometallurgy, vol 71, pp. 329-334, 2004. DOI: https://doi.org/10.1016/S0304-386X(03)00082-3 DOI: https://doi.org/10.1016/S0304-386X(03)00082-3
D. Dreisinger, “Copper leaching from primary sulfides: Options for biological and chemical extraction of copper,” Hydrometallurgy, vol. 83, no. 1–4, pp. 10–20, Sep. 2006. DOI: 10.1016/j.hydromet.2006.03.032. DOI: https://doi.org/10.1016/j.hydromet.2006.03.032
A. R. Burkin, Solid-state transformations during leaching. Min Sci. Eng. 1. 1969.
E. M. Córdoba, J. A. Muñoz, M. L. Blázquez, F. González, and A. Ballester, “Leaching of chalcopyrite with ferric ion. Part I: General aspects,” Hydrometallurgy, vol. 93, no. 3–4, pp. 81–87, Aug. 2008. DOI: 10.1016/j.hydromet.2008.04.015. DOI: https://doi.org/10.1016/j.hydromet.2008.04.015
V. J. Martínez-Gómez, J. C. Fuentes-Aceituno, R. Pérez-Garibay, and J. C. Lee, “A phenomenological study of the electro-assisted reductive leaching of chalcopyrite,” Hydrometallurgy, vol. 164, pp. 54–63, Sep. 2016. DOI: 10.1016/j.hydromet.2016.05.008. DOI: https://doi.org/10.1016/j.hydromet.2016.05.008
V. J. Martínez-Gómez, J. C. Fuentes-Aceituno, R. Pérez-Garibay, and J. chun Lee, “A study of the electro-assisted reductive leaching of a chalcopyrite concentrate in HCl solutions. Part I: Kinetic behavior and nature of the chalcopyrite reduction,” Hydrometallurgy, vol. 181, pp. 195–205, Nov. 2018. DOI: 10.1016/j.hydromet.2018.09.012. DOI: https://doi.org/10.1016/j.hydromet.2018.09.012
P. B. Munoz, J. D. Miller, and M. E. Wadsworth, “Reaction Mechanism for the Acid Ferric Sulfate Leaching of Chalcopyrite,” Metallurgical Transactions B, pp. 149–158, 1979. DOI: https://doi.org/10.1007/BF02652458. DOI: https://doi.org/10.1007/BF02652458
D. Dreisinger and N. Abed, “A fundamental study of the reductive leaching of chalcopyrite using metallic iron part I: kinetic analysis,” Hydrometallurgy, pp. 37–57, 2002. DOI:10.1016/S0304-386X(02)00079-8. DOI: https://doi.org/10.1016/S0304-386X(02)00079-8
F. Doyle and G. Lapidus, “Reductive Leaching of Chalcopyrite by Aluminum,” ECS Trans, vol. 2, no. 3, pp. 189–196, Apr. 2006. DOI: 10.1149/1.2196009. DOI: https://doi.org/10.1149/1.2196009
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 EPISTEMUS

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The magazine acquires the patrimonial rights of the articles only for diffusion without any purpose of profit, without diminishing the own rights of authorship.
The authors are the legitimate owners of the intellectual property rights of their respective articles, and in such quality, by sending their texts they express their desire to collaborate with the Epistemus Magazine, published biannually by the University of Sonora.
Therefore, freely, voluntarily and free of charge, once accepted the article for publication, they give their rights to the University of Sonora for the University of Sonora to edit, publish, distribute and make available through intranets, Internet or CD said work, without any limitation of form or time, as long as it is non-profit and with the express obligation to respect and mention the credit that corresponds to the authors in any use that is made of it.
It is understood that this authorization is not an assignment or transmission of any of your economic rights in favor of the said institution. The University of Sonora guarantees the right to reproduce the contribution by any means in which you are the author, subject to the credit being granted corresponding to the original publication of the contribution in Epistemus.
Unless otherwise indicated, all the contents of the electronic edition are distributed under a license for use and Creative Commons — Attribution-NonCommercial-ShareAlike 4.0 International — (CC BY-NC-SA 4.0) You can consult here the informative version and the legal text of the license. This circumstance must be expressly stated in this way when necessary.
The names and email addresses entered in this journal will be used exclusively for the purposes established in it and will not be provided to third parties or for their use for other purposes.