Comprehensive Analysis of the Dissolution of Precious Metals with Innovative Amine-Based Leaching Systems
DOI:
https://doi.org/10.36790/epistemus.v17i34.267Keywords:
Monoethanolamine, ethylamine, oxidation, leaching, precious metalsAbstract
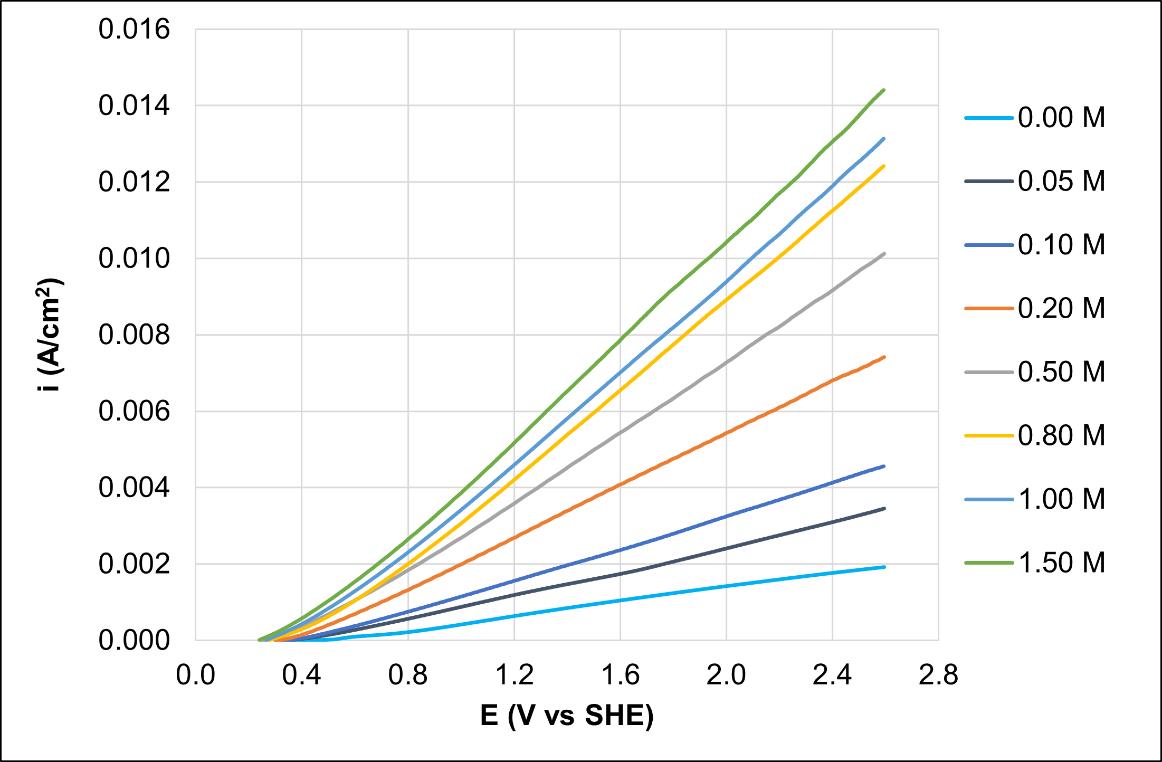
In this research work, novel amine-based leaching systems monoethanolamine (MEA) and ethylamine (EA) were studied from an electrochemical approach to improve the understanding of the dissolution reaction mechanism of precious metals, i.e., gold and silver. The effect of MEA and EA concentration on the oxidation rate of precious metals is presented. The study is complemented with electrochemical techniques such as: open circuit potential technique (OCP), linear voltammetry (VL) and chronoamperometry. The results show that these amines have a great potential in dissolving Au and Ag since average current densities of 0.008 and 0.013 A/cm2 are reached using the MEA, and 0.023 and 0.029 A/cm2 using the EA, respectively. Furthermore, the results indicate that both MEA and EA could decrease the environmental impact involved in hydrometallurgical processes.
Downloads
References
W. D. Callister, Fundamentals of Materials Science and Engineering, Fifth Edit. 2001.
J. Cui and L. Zhang, “Metallurgical recovery of metals from electronic waste : A review,” vol. 158, pp. 228–256, 2008, doi: 10.1016/j.jhazmat.2008.02.001. DOI: https://doi.org/10.1016/j.jhazmat.2008.02.001
C. Hagelüeken, “Recycling of Electronic Scrap At Umicore Precious Metals,” Acta Metall. Slovaca, vol. 12, no. January, pp. 111–120, 2006.
M. G. Aylmore and D. M. Muir, “Thiosulfate leaching of gold - a review,” Miner. Eng., vol. 14, no. 2, pp. 135–174, 2001, doi: 10.1016/S0892-6875(00)00172-2. DOI: https://doi.org/10.1016/S0892-6875(00)00172-2
E. Reyes-Sandoval and J. C. Fuentes-Aceituno, “Maximization of the silver recovery with the ‘monoethanolamine-copper-ammonium sulfate’ novel system. A step towards the development of a less toxic leaching technology,” Hydrometallurgy, vol. 173, no. June, pp. 22–31, 2017, doi: 10.1016/j.hydromet.2017.07.007. DOI: https://doi.org/10.1016/j.hydromet.2017.07.007
A. Holguin-Gonzalez, J. C. Fuentes-Aceituno, M. seuk Kim, and J. chun Lee, “A kinetic-mechanistic study of silver oxidation with the NaNO2–CuSO4 alternative novel system,” Electrochim. Acta, vol. 337, no. 2, p. 135792, 2020, doi: 10.1016/j.electacta.2020.135792. DOI: https://doi.org/10.1016/j.electacta.2020.135792
D. M. Puente-Siller, J. C. Fuentes-Aceituno, and F. Nava-Alonso, “An analysis of the efficiency and sustainability of the thiosulfate-copper-ammonia-monoethanolamine system for the recovery of silver as an alternative to cyanidation,” Hydrometallurgy, vol. 169, pp. 16–25, 2017, doi: 10.1016/j.hydromet.2016.12.003. DOI: https://doi.org/10.1016/j.hydromet.2016.12.003
D. M. Puente-Siller, J. C. Fuentes-Aceituno, and F. Nava-Alonso, “Hydrometallurgy A kinetic – thermodynamic study of silver leaching in thiosulfate – copper – ammonia – EDTA solutions,” Hydrometallurgy, vol. 134–135, pp. 124–131, 2013, doi: 10.1016/j.hydromet.2013.02.010. DOI: https://doi.org/10.1016/j.hydromet.2013.02.010
D. M. Puente-Siller, J. C. Fuentes-Aceituno, and F. Nava-Alonso, “Study of thiosulfate leaching of silver sulfide in the presence of EDTA and sodium citrate. Effect of NaOH and NH4OH,” Hydrometallurgy, vol. 149, pp. 1–11, 2014, doi: 10.1016/j.hydromet.2014.06.004. DOI: https://doi.org/10.1016/j.hydromet.2014.06.004
S. D. Sharma and M. Azzi, “A critical review of existing strategies for emission control in the monoethanolamine-based carbon capture process and some recommendations for improved strategies,” Fuel, vol. 121, pp. 178–188, 2014, doi: 10.1016/j.fuel.2013.12.023. DOI: https://doi.org/10.1016/j.fuel.2013.12.023
M. A. Sutton, J. W. Erisman, F. Dentener, and D. Möller, “Ammonia in the environment: From ancient times to the present,” Environ. Pollut., vol. 156, no. 3, pp. 583–604, 2008, doi: 10.1016/j.envpol.2008.03.013. DOI: https://doi.org/10.1016/j.envpol.2008.03.013
C. E. Cos Castillo, “Estudio electroquímico del comportamiento redox de la plata en sistemas acuosos sin cianuro,” Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, 2020.
C. E. Cos-Castillo and J. C. Fuentes-Aceituno, “A comprehensive mechanistic analysis of silver dissolution with the monoethanolamine-copper-ammonium system and the development of a novel leaching technology,” Miner. Eng., vol. 186, p. 107753, Aug. 2022, doi: 10.1016/J.MINENG.2022.107753. DOI: https://doi.org/10.1016/j.mineng.2022.107753
R. M. Smith and A. E. Martell, Critical Stability Constants Volumen 2: Amines, vol. 2. 1975. DOI: https://doi.org/10.1007/978-1-4613-4452-0
M. M. Aghamirian and W. T. Yen, “A study of gold anodic behavior in the presence of various ions and sulfide minerals in cyanide solution,” Miner. Eng., vol. 18, no. 1, pp. 89–102, 2005, doi: 10.1016/j.mineng.2004.05.008. DOI: https://doi.org/10.1016/j.mineng.2004.05.008
J. Li and M. E. Wadsworth, “Electrochemical Study of Silver Dissolution in Cyanide Solutions,” J. Electrochem. Soc., vol. 140, no. 7, pp. 1921–1927, 1993, doi: 10.1149/1.2220740. DOI: https://doi.org/10.1149/1.2220740
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 EPISTEMUS

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The magazine acquires the patrimonial rights of the articles only for diffusion without any purpose of profit, without diminishing the own rights of authorship.
The authors are the legitimate owners of the intellectual property rights of their respective articles, and in such quality, by sending their texts they express their desire to collaborate with the Epistemus Magazine, published biannually by the University of Sonora.
Therefore, freely, voluntarily and free of charge, once accepted the article for publication, they give their rights to the University of Sonora for the University of Sonora to edit, publish, distribute and make available through intranets, Internet or CD said work, without any limitation of form or time, as long as it is non-profit and with the express obligation to respect and mention the credit that corresponds to the authors in any use that is made of it.
It is understood that this authorization is not an assignment or transmission of any of your economic rights in favor of the said institution. The University of Sonora guarantees the right to reproduce the contribution by any means in which you are the author, subject to the credit being granted corresponding to the original publication of the contribution in Epistemus.
Unless otherwise indicated, all the contents of the electronic edition are distributed under a license for use and Creative Commons — Attribution-NonCommercial-ShareAlike 4.0 International — (CC BY-NC-SA 4.0) You can consult here the informative version and the legal text of the license. This circumstance must be expressly stated in this way when necessary.
The names and email addresses entered in this journal will be used exclusively for the purposes established in it and will not be provided to third parties or for their use for other purposes.