Chalcopyrite Leaching with Ethylenediaminetetraacetic Acid (EDTA) in Oxidizing Medium
DOI:
https://doi.org/10.36790/epistemus.v17i34.274Keywords:
Chalcopyrite, Hydrometallurgy, Leaching, EDTAAbstract
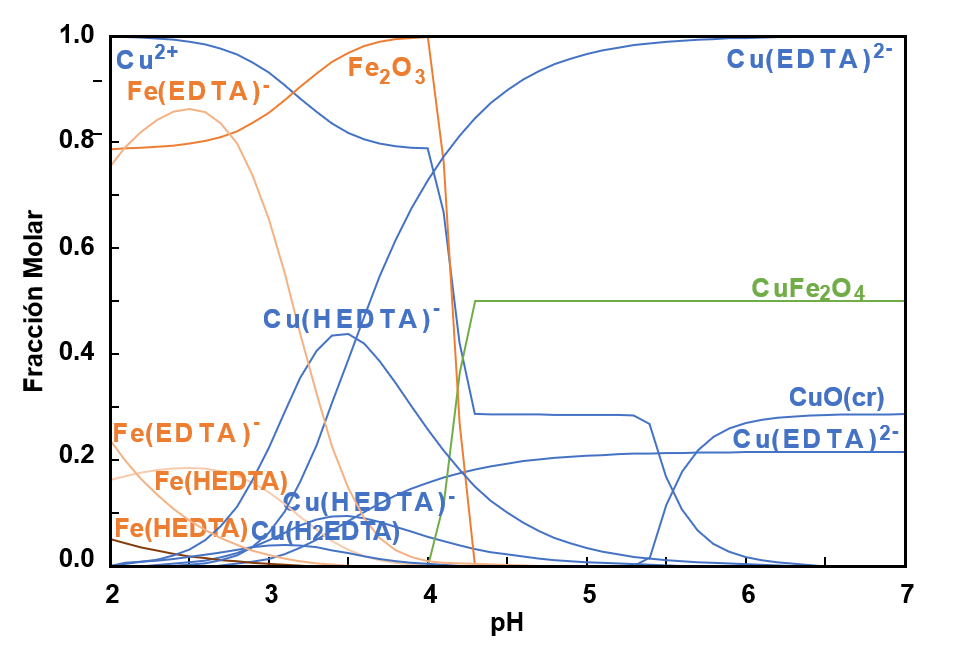
In nature, copper is presented predominantly as sulfide complexes, chalcopyrite (CuFeS2), bornite (Cu5FeS4), and chalcocite (Cu2S). Copper recovery from chalcopyrite by leaching is difficult due to chemical stability and involves using complex systems for long-term treatment. In the present work, an alternative for leaching chalcopyrite under controlled conditions is presented to evaluate the effect of pH, EDTA concentration, and hydrogen peroxide on copper recovery. With the help of a factorial design, the aim is to optimize the copper recovery process with less iron dissolution. According to the experimental results, a low concentration of EDTA (0.0006 M) promotes the selective dissolution of copper (20.04%) and low dissolution of iron (0.2%) at pH 4.5 and room temperature.
Downloads
References
Anyadike, N. (2002). 1 - Background: key issues. In N. Anyadike (Ed.), Copper (pp. 1-26): Woodhead Publishing. https://doi.org/10.1016/B978-1-85573-592-7.50004-7. DOI: https://doi.org/10.1016/B978-1-85573-592-7.50004-7
A. Elshkaki, T. Gradel, L. Ciacci, B.K. Reck, “Copper demand, supply, and associated energy use to 2050”, Global Environmental Change, vol 39, pp 305-315, Junio 2016. https://doi:10.1016/j.gloenvcha.2016.06.006 DOI: https://doi.org/10.1016/j.gloenvcha.2016.06.006
Z. Liu, Z. Yin, H. Hu, Q.Y. Chen, “Leaching kinetics of low-grade copper ore with high-alkality gangues in ammonia-ammonium sulphate solution”, J. Cent. South Univ. Technol, vol 19, pp 77-84, January 2012. http://doi:10.1007/s11771-012-0975-8 DOI: https://doi.org/10.1007/s11771-012-0975-8
Y. Li, N. Kawashima, J. Li, A.P. Chandra, A.R. Gerson, “A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite”, Advances in Colloid and Interface Science, vol 197–198, Marzo 2013, Pages 1-32, https://doi.org/10.1016/j.cis.2013.03.004. DOI: https://doi.org/10.1016/j.cis.2013.03.004
Pradhan, N., Nathsarma, K. C., Srinivasa Rao, K., Sukla, L. B., & Mishra, B. K. (2008). Heap bioleaching of chalcopyrite: A review. Minerals Engineering, 21(5), 355-365. https://doi:10.1016/j.mineng.2007.10.018. DOI: https://doi.org/10.1016/j.mineng.2007.10.018
I.F. Barton, J. Brent, “Chalcopyrite leaching in novel lixiviants”, Hydrometallurgy, vol. 207, pp 20, November 2021 https://doi.org/10.1016/j.hydromet.2021.105775 DOI: https://doi.org/10.1016/j.hydromet.2021.105775
F. Habashi, “Processes for Sulfur Recovery from ores”, American Mining Congress, Washington, D.C, 1969, pp 12.
R. Romero, A Mazuelos, I. Palencia, F. Carranza. “Copper recovery from chalcopyrite concentrates by the BRISA process”, Hydrometallurgy, vol 70, pp 205-215, March 2003. https://doi.org/10.1016/S0304-386X(03)00081-1. DOI: https://doi.org/10.1016/S0304-386X(03)00081-1
Moyo, T., & Petersen, J. (2016). Study of the dissolution of chalcopyrite in solutions of different ammonium salts. Journal of the Southern African Institute of Mining and Metallurgy, 116, 509-516. http://doi:10.17159/2411-9717/2016/v116n6a4. DOI: https://doi.org/10.17159/2411-9717/2016/v116n6a4
Y. Ma, Y. Yang, R. Fan, X. Gao, l. Zheng, M. Chen.” Chalcopyrite leaching in ammonium chloride solutions under ambient conditions: Insight into the dissolution mechanism by XANES, Raman spectroscopy and electrochemical studies”, Elsevier, pp 13, June 2021. https://doi:10.1016/j.mineng.2021.107063. DOI: https://doi.org/10.1016/j.mineng.2021.107063
T.C. Chagas, E.M. Garcia, H.W. Alves, T. Matencio, V. F. Cunha, “Electrochemical study of chalcopyrite dissolution in sulfuric, nitric and hydrochloric acid solutions”. International Journal of Mineral Processing, vol. 49, pp. 25-33, February 2016. https://doi:10.1016/j.minpro.2016.02.001. DOI: https://doi.org/10.1016/j.minpro.2016.02.001
Ruiz-Sánchez, A., Lázaro, I., & Lapidus, G. T. (2020). Improvement effect of organic ligands on chalcopyrite leaching in the aqueous medium of sulfuric acid hydrogen peroxide-ethylene glycol. Hydrometallurgy, 193, 105293. https://doi.org/10.1016/j.hydromet.2020.105293 DOI: https://doi.org/10.1016/j.hydromet.2020.105293
Abdullah, M. A. M., Seman, M. N. A., Chik, S. M. S. T., & Abdullah, S. B. (2022). Factorial design in optimizing parameters for thermoresponsive ionic liquids as draw solution. Process Safety and Environmental Protection, 161, 34-49. https://doi.org/10.1016/j.psep.2022.03.016 DOI: https://doi.org/10.1016/j.psep.2022.03.016
Hamdi, M. M., Thiru, S., Gzara, L., & Bin Mahfouz, A. S. (2018). Application of factorial design for modeling reverse osmosis process using thin film composite polyamide membrane: A theoretical analysis and experimental validation. Desalination and Water Treatment, 124, 37-52. https://doi:10.5004/dwt.2018.22749 DOI: https://doi.org/10.5004/dwt.2018.22749
Cacua, K., Buitrago-Sierra, R., Herrera, B., Chejne, F., & Pabón, E. (2017). Influence of different parameters and their coupled effects on the stability of alumina nanofluids by a fractional factorial design approach. Advanced Powder Technology, 28(10), 2581-2588. https://doi.org/10.1016/j.apt.2017.07.009 DOI: https://doi.org/10.1016/j.apt.2017.07.009
P. B. Munoz, J. D. Miller, and M. E. Wadsworth, "Reaction mechanism for the acid ferric sulfate leaching of chalcopyrite," Metallurgical Transactions B, vol. 10, no. 2, pp. 149-158, 1979/06/01 1979. https://doi.org/10.1007/BF02652458 DOI: https://doi.org/10.1007/BF02652458
Puigdomenech, I. (2004). Make equilibrium diagrams using sophisticated algorithms (MEDUSA), inorganic chemistry. Royal Institute of Technology.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 EPISTEMUS

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The magazine acquires the patrimonial rights of the articles only for diffusion without any purpose of profit, without diminishing the own rights of authorship.
The authors are the legitimate owners of the intellectual property rights of their respective articles, and in such quality, by sending their texts they express their desire to collaborate with the Epistemus Magazine, published biannually by the University of Sonora.
Therefore, freely, voluntarily and free of charge, once accepted the article for publication, they give their rights to the University of Sonora for the University of Sonora to edit, publish, distribute and make available through intranets, Internet or CD said work, without any limitation of form or time, as long as it is non-profit and with the express obligation to respect and mention the credit that corresponds to the authors in any use that is made of it.
It is understood that this authorization is not an assignment or transmission of any of your economic rights in favor of the said institution. The University of Sonora guarantees the right to reproduce the contribution by any means in which you are the author, subject to the credit being granted corresponding to the original publication of the contribution in Epistemus.
Unless otherwise indicated, all the contents of the electronic edition are distributed under a license for use and Creative Commons — Attribution-NonCommercial-ShareAlike 4.0 International — (CC BY-NC-SA 4.0) You can consult here the informative version and the legal text of the license. This circumstance must be expressly stated in this way when necessary.
The names and email addresses entered in this journal will be used exclusively for the purposes established in it and will not be provided to third parties or for their use for other purposes.