Recent Applications of Chlorophyll Fluorescence in Plant Crops
DOI:
https://doi.org/10.36790/epistemus.v16i33.285Keywords:
Chlorophyll a, climate change, phytopathogensAbstract
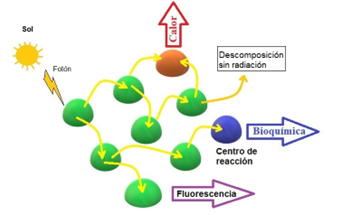
The production of agricultural crops in the face of climate change is a topic of current interest. In recent years, the negative effects of biotic and abiotic factors on its productivity have been observed. In this sense, the clarification of the tolerance mechanisms that plants use towards the various stress factors should be outlined as a strategy to generate cultivars and resilient production systems. The measurement of chlorophyll fluorescence is a fast and non-destructive way to understand the sensitivity of plants to various factors. This article jointly reviews scientific information about the effects of changing abiotic and biotic factors that are currently recorded on the chlorophyll fluorescence of plants crops. The utility of fluorescence parameters as indicators of adaptation mechanisms and future trends in their use are discussed.
Downloads
References
A. Stirbet, D. Lazár, J. Kromdijk, and Govindjee, “Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses?,” Photosynthetica, vol. 56, no. 1, pp. 86–104, Mar. 2018, doi: 10.1007/S11099-018-0770-3. DOI: https://doi.org/10.1007/s11099-018-0770-3
M. R. Urschel and T. Pocock, “Remote detection of growth dynamics in red lettuce using a novel chlorophyll a fluorometer,” Agronomy, vol. 8, no. 10, Oct. 2018, doi: 10.3390/AGRONOMY8100227. DOI: https://doi.org/10.3390/agronomy8100227
B. Cremella, Y. Huot, and S. Bonilla, “Interpretation of total phytoplankton and cyanobacteria fluorescence from cross-calibrated fluorometers, including sensitivity to turbidity and colored dissolved organic matter,” Limnol. Oceanogr.: Methods, vol. 16, pp. 881–894, 2018, doi: 10.1002/lom3.10290. DOI: https://doi.org/10.1002/lom3.10290
S. I. Zandalinas, Y. Fichman, and R. Mittler, “Vascular Bundles Mediate Systemic Reactive Oxygen Signaling during Light Stress,” Plant Cell, vol. 32, no. 11, pp. 3425–3435, Nov. 2020, doi: 10.1105/TPC.20.00453. DOI: https://doi.org/10.1105/tpc.20.00453
J. Banks and J. M. Banks, “Identification of Drought Tolerant Amenity Trees,” Environ Exp Bot, vol. 155, pp. 118–127, 2018, Accessed: Dec. 07, 2022. [Online]. Available: https://www.researchgate.net/publication/335110849 DOI: https://doi.org/10.1016/j.envexpbot.2018.06.022
B. Liu, J. Liang, G. Tang, X. Wang, F. Liu, and D. Zhao, “Drought stress affects on growth, water use efficiency, gas exchange and chlorophyll fluorescence of Juglans rootstocks,” Sci Hortic, vol. 250, pp. 230–235, May 2019, doi: 10.1016/J.SCIENTA.2019.02.056. DOI: https://doi.org/10.1016/j.scienta.2019.02.056
R. Zhou et al., “Evaluation of temperature stress tolerance in cultivated and wild tomatoes using photosynthesis and chlorophyll fluorescence,” Hortic Environ Biotechnol, vol. 4, no. 59, pp. 499–509, Aug. 2018, doi: 10.1007/S13580-018-0050-Y. DOI: https://doi.org/10.1007/s13580-018-0050-y
M. Stefanov, E. Yotsova, G. Rashkov, K. Ivanova, Y. Markovska, and E. L. Apostolova, “Effects of salinity on the photosynthetic apparatus of two Paulownia lines,” Plant Physiology and Biochemistry, vol. 101, pp. 54–59, Apr. 2016, doi: 10.1016/J.PLAPHY.2016.01.017. DOI: https://doi.org/10.1016/j.plaphy.2016.01.017
Y. Zhang and G. jian Liu, “Effects of cesium accumulation on chlorophyll content and fluorescence of Brassica juncea L.,” J Environ Radioact, vol. 195, pp. 26–32, Dec. 2018, doi: 10.1016/j.jenvrad.2018.09.017. DOI: https://doi.org/10.1016/j.jenvrad.2018.09.017
M. L. Pérez-Bueno, M. Pineda, and M. Barón, “Phenotyping Plant Responses to Biotic Stress by Chlorophyll Fluorescence Imaging,” Frontiers in Plant Science, vol. 10. Frontiers Media S.A., Sep. 18, 2019. doi: 10.3389/fpls.2019.01135. DOI: https://doi.org/10.3389/fpls.2019.01135
N. R. Baker and E. Rosenqvist, “Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities,” Journal of Experimental Botany, vol. 55, no. 403. Oxford University Press, pp. 1607–1621, 2004. doi: 10.1093/jxb/erh196. DOI: https://doi.org/10.1093/jxb/erh196
X. G. Zhu, Govindjee, N. R. Baker, E. DeSturler, D. R. Ort, and S. P. Long, “Chlorophyll a fluorescence induction kinetics in leaves predicted from a model describing each discrete step of excitation energy and electron transfer associated with Photosystem II,” Planta, vol. 223, no. 1, pp. 114–133, Dec. 2005, doi: 10.1007/s00425-005-0064-4. DOI: https://doi.org/10.1007/s00425-005-0064-4
K. Roháček, “Chlorophyll Fluorescence Parameters: The Definitions, Photosynthetic Meaning, and Mutual Relationships,” Photosynthetica, vol. 40, no. 13, pp. 13–29, 2002, doi: 10.1023/A:1020125719386. DOI: https://doi.org/10.1023/A:1020125719386
M. F. Seleiman et al., “Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects,” Plants 2021, Vol. 10, Page 259, vol. 10, no. 2, p. 259, Jan. 2021, doi: 10.3390/PLANTS10020259. DOI: https://doi.org/10.3390/plants10020259
A. K. Shanker et al., “Drought stress responses in crops,” Functional and Integrative Genomics, vol. 14, no. 1. Springer Verlag, pp. 11–22, 2014. doi: 10.1007/s10142-013-0356-x. DOI: https://doi.org/10.1007/s10142-013-0356-x
Y. K. Shin, S. R. Bhandari, J. S. Jo, J. W. Song, and J. G. Lee, “Effect of drought stress on chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities in lettuce seedlings,” Horticulturae, vol. 7, no. 8, Aug. 2021, doi: 10.3390/horticulturae7080238. DOI: https://doi.org/10.3390/horticulturae7080238
X. Yang et al., “Photosynthetic Response Mechanism of Soil Salinity-Induced Cross-Tolerance to Subsequent Drought Stress in Tomato Plants,” Plants (Basel), vol. 9, no. 3, Mar. 2020, doi: 10.3390/PLANTS9030363. DOI: https://doi.org/10.3390/plants9030363
V. K. Dalal and B. C. Tripathy, “Water-stress induced downsizing of light-harvesting antenna complex protects developing rice seedlings from photo-oxidative damage OPEN,” Sci Rep, vol. 8, no. 1, pp. 1–16, 2018, doi: 10.1038/s41598-017-14419-4. DOI: https://doi.org/10.1038/s41598-017-14419-4
P. Parihar, S. Singh, R. Singh, V. P. Singh, and S. M. Prasad, “Effect of salinity stress on plants and its tolerance strategies: a review,” Environmental Science and Pollution Research, vol. 22, no. 6, pp. 4056–4075, Mar. 2015, doi: 10.1007/s11356-014-3739-1. DOI: https://doi.org/10.1007/s11356-014-3739-1
H. Hnilickova, K. Kraus, P. Vachova, F. Hnilicka, P. Woodrow, and L. F. Ciarmiello, “Salinity Stress Affects Photosynthesis, Malondialdehyde Formation, and Proline Content in Portulaca oleracea L,” Plants, vol. 10, p. 845, 2021, doi: 10.3390/plants10050845. DOI: https://doi.org/10.3390/plants10050845
R. Gholamin and M. Khayatnezhad, “Study of Bread Wheat Genotype Physiological and Biochemical Responses to Drought Stress,” HELIX, vol. 10, no. 5, pp. 87–92, Oct. 2020, doi: 10.29042/2020-10-5-87-92. DOI: https://doi.org/10.29042/2020-10-5-87-92
D. Allel, A. Ben-Amar, and C. Abdelly, “Leaf photosynthesis, chlorophyll fluorescence and ion content of barley (Hordeum vulgare) in response to salinity,” https://doi.org/10.1080/01904167.2017.1385811, vol. 41, no. 4, pp. 497–508, Feb. 2017, doi: 10.1080/01904167.2017.1385811. DOI: https://doi.org/10.1080/01904167.2017.1385811
M. S. Saddiq et al., “Effect of salinity stress on physiological changes in winter and spring wheat,” Agronomy, vol. 11, no. 6, Jun. 2021, doi: 10.3390/agronomy11061193. DOI: https://doi.org/10.3390/agronomy11061193
T. Starman and L. Lombardini, “Growth, Gas Exchange, and Chlorophyll Fluorescence of Four Ornamental Herbaceous Perennials during Water Deficit Conditions,” Journal of the American Society for Horticultural Science, vol. 131, no. 4, pp. 469–475, Jul. 2006, doi: 10.21273/JASHS.131.4.469. DOI: https://doi.org/10.21273/JASHS.131.4.469
X. Song et al., “Nitrogen Application Improved Photosynthetic Productivity, Chlorophyll Fluorescence, Yield and Yield Components of Two Oat Genotypes under Saline Conditions,” Agronomy 2019, Vol. 9, Page 115, vol. 9, no. 3, p. 115, Feb. 2019, doi: 10.3390/AGRONOMY9030115. DOI: https://doi.org/10.3390/agronomy9030115
M. Shahid et al., “Heavy metal stress and crop productivity,” Crop Production and Global Environmental Issues, pp. 1–25, Jan. 2015, doi: 10.1007/978-3-319-23162-4_1/COVER. DOI: https://doi.org/10.1007/978-3-319-23162-4_1
J. Fan et al., “Physiological effects induced by aluminium and fluoride stress in tall fescue (Festuca arundinacea Schreb),” Ecotoxicol Environ Saf, vol. 231, Feb. 2022, doi: 10.1016/J.ECOENV.2022.113192. DOI: https://doi.org/10.1016/j.ecoenv.2022.113192
A. Dezhban, • A Shirvany, • P Attarod, • M Delshad, • M Matinizadeh, and • M Khoshnevis, “Cadmium and lead effects on chlorophyll fluorescence, chlorophyll pigments and proline of Robinia pseudoacacia,” J For Res (Harbin), vol. 26, no. 2, pp. 323–329, 2015, doi: 10.1007/s11676-015-0045-9. DOI: https://doi.org/10.1007/s11676-015-0045-9
D. Killi, A. Raschi, and F. Bussotti, “Lipid Peroxidation and Chlorophyll Fluorescence of Photosystem II Performance during Drought and Heat Stress is Associated with the Antioxidant Capacities of C3 Sunflower and C4 Maize Varieties,” International Journal of Molecular Science, vol. 21, no. 14, p. 4846, 2020, doi: 10.3390/ijms21144846. DOI: https://doi.org/10.3390/ijms21144846
S. E. Bruce, D. B. Rowe, and J. A. Flore, “Chlorophyll Fluorescence and Vegetative Propagation of Taxus,” HortScience, vol. 36, no. 5, pp. 971–975, Aug. 2001, doi: 10.21273/HORTSCI.36.5.971. DOI: https://doi.org/10.21273/HORTSCI.36.5.971
E. Zareei et al., “Physiological and biochemical responses of strawberry crown and leaf tissues to freezing stress,” BMC Plant Biol, vol. 21, no. 1, pp. 1–17, Dec. 2021, doi: 10.1186/S12870-021-03300-2/TABLES/4. DOI: https://doi.org/10.1186/s12870-021-03300-2
R. Shahzad et al., “Comparative analysis of two phytochrome mutants of tomato (Micro-Tom cv.) reveals specific physiological, biochemical, and molecular responses under chilling stress,” Journal of Genetic Engineering and Biotechnology, vol. 18, no. 1, Dec. 2020, doi: 10.1186/s43141-020-00091-1. DOI: https://doi.org/10.1186/s43141-020-00091-1
J. Miralles-Crespo, J. Antonio Martínez-López, J. Antonio Franco-Leemhuis, and S. Bañ, “Determining Freezing Injury from Changes in Chlorophyll Fluorescence in Potted Oleander Plants,” Wilson and Greaves, 2011. DOI: https://doi.org/10.21273/HORTSCI.46.6.895
R. Cassia, M. Nocioni, N. Correa-Aragunde, and L. Lamattina, “Climate change and the impact of greenhouse gasses: CO2 and NO, friends and foes of plant oxidative stress,” Frontiers in Plant Science, vol. 9. Frontiers Media S.A., Mar. 01, 2018. doi: 10.3389/fpls.2018.00273. DOI: https://doi.org/10.3389/fpls.2018.00273
D. R. Taub, J. R. Seemann, and J. S. Coleman, “Growth in elevated CO2 protects photosynthesis against high-temperature damage,” Plant Cell Environ, vol. 23, no. 6, pp. 649–656, Jun. 2000, doi: 10.1046/J.1365-3040.2000.00574.X. DOI: https://doi.org/10.1046/j.1365-3040.2000.00574.x
E. van Tongerlo, G. Trouwborst, S. W. Hogewoning, W. van Ieperen, J. A. Dieleman, and L. F. M. Marcelis, “Crassulacean acid metabolism species differ in the contribution of C3 and C4 carboxylation to end of day CO2 fixation,” Physiol Plant, vol. 172, no. 1, pp. 134–145, May 2021, doi: 10.1111/PPL.13312. DOI: https://doi.org/10.1111/ppl.13312
X. Zhao et al., “Elevated CO 2 concentration promotes photosynthesis of grape (Vitis vinifera L. cv. ’Pinot noir’) plantlet in vitro by regulating RbcS and Rca revealed by proteomic and transcriptomic profiles,” BMC Plant Biol, vol. 19, no. 1, pp. 1–16, Jan. 2019, doi: 10.1186/S12870-019-1644-Y/FIGURES/5. DOI: https://doi.org/10.1186/s12870-019-1644-y
X. Liu et al., “Increased CO2 concentrations increasing water use efficiency and improvement PSII function of mulberry seedling leaves under drought stress,” J Plant Interact, vol. 14, no. 1, pp. 213–223, Jan. 2019, doi: 10.1080/17429145.2019.1603405. DOI: https://doi.org/10.1080/17429145.2019.1603405
M. S. Hunjan and J. S. Lore, “Climate Change: Impact on Plant Pathogens, Diseases, and Their Management,” Crop Protection Under Changing Climate, pp. 85–100, 2020, doi: 10.1007/978-3-030-46111-9_4. DOI: https://doi.org/10.1007/978-3-030-46111-9_4
M. Kopacki, A. Wagner, and W. Michałek, “PATHOGENICITY OF Fusarium oxysporum, Fusarium avenaceum AND Sclerotinia sclerotiorum AND THEIR EFFECT ON PHOTOSYNTHETIC ACTIVITY OF CHRYSANTHEMUM PLANTS,” Acta Sci. Pol. Hortorum Cultus, vol. 15, no. 3, pp. 59–70, 2016, Accessed: Dec. 07, 2022. [Online]. Available: www.acta.media.pl
K. Mandal, R. Saravanan, S. Maiti, and I. L. Kothari, “Effect of downy mildew disease on photosynthesis and chlorophyll fluorescence in Plantago ovata Forsk. Einfluss des Falschen Mehltaus auf Photosynthese und Chlorophyllfluoreszenz von Plantago ovata Forsk,” Journal of Plant Diseases and Protection, vol. 116, no. 4, pp. 1861–3829, 2009. DOI: https://doi.org/10.1007/BF03356305
C. E. Aucique-Pérez, P. E. de Menezes Silva, W. R. Moreira, F. M. DaMatta, and F. Á. Rodrigues, “Photosynthesis impairments and excitation energy dissipation on wheat plants supplied with silicon and infected with Pyricularia oryzae,” Plant Physiology and Biochemistry, vol. 121, pp. 196–205, Dec. 2017, doi: 10.1016/J.PLAPHY.2017.10.023. DOI: https://doi.org/10.1016/j.plaphy.2017.10.023
L. Wang, S. Poque, and J. P. T. Valkonen, “Phenotyping viral infection in sweetpotato using a high-throughput chlorophyll fluorescence and thermal imaging platform.,” Plant Methods, p. NA-NA, Oct. 2019, Accessed: Dec. 08, 2022. [Online]. Available: https://go.gale.com/ps/i.do?p=HRCA&sw=w&issn=17464811&v=2.1&it=r&id=GALE%7CA604534220&sid=googleScholar&linkaccess=fulltext DOI: https://doi.org/10.1186/s13007-019-0501-1
P. Spoustová, H. Synková, R. Valcke, and N. Čeřovská, “Chlorophyll a fluorescence as a tool for a study of the Potato virus Y effects on photosynthesis of nontransgenic and transgenic Pssu-ipt tobacco,” Photosynthetica, vol. 51, no. 2, pp. 191–201, Jun. 2013, doi: 10.1007/S11099-013-0023-4. DOI: https://doi.org/10.1007/s11099-013-0023-4
J. Kuckenberg, I. Tartachnyk, and G. Noga, “Temporal and spatial changes of chlorophyll fluorescence as a basis for early and precise detection of leaf rust and powdery mildew infections in wheat leaves,” Precis Agric, vol. 10, no. 1, pp. 34–44, Feb. 2009, doi: 10.1007/S11119-008-9082-0. DOI: https://doi.org/10.1007/s11119-008-9082-0
B. Y. Samaniego-Gámez, R. Garruña, J. M. Tun-Suárez, J. Kantun-Can, A. Reyes-Ramírez, and L. Cervantes-Díaz, “Bacillus spp. Inoculation improves photosystem II efficiency and enhances photosynthesis in pepper plants,” Chil J Agric Res, vol. 76, no. 4, pp. 409–416, 2016, doi: 10.4067/S0718-58392016000400003. DOI: https://doi.org/10.4067/S0718-58392016000400003
J. Reimer et al., “An autonomous and wireless pulse-amplitude modulated chlorophyll fluorometer,” Technisches Messen, vol. 88, no. 12, pp. 773–784, Dec. 2021, doi: 10.1515/TEME-2021-0104/MACHINEREADABLECITATION/RIS. DOI: https://doi.org/10.1515/teme-2021-0104
L. He et al., “Diverse photosynthetic capacity of global ecosystems mapped by satellite chlorophyll fluorescence measurements,” Remote Sens Environ, vol. 232, p. 111344, Oct. 2019, doi: 10.1016/J.RSE.2019.111344. DOI: https://doi.org/10.1016/j.rse.2019.111344
L. L. Sloat et al., “Evaluating the benefits of chlorophyll fluorescence for in-season crop productivity forecasting,” Remote Sens Environ, vol. 260, p. 112478, Jul. 2021, doi: 10.1016/J.RSE.2021.112478. DOI: https://doi.org/10.1016/j.rse.2021.112478
G. H. Mohammed et al., “Remote sensing of solar-induced chlorophyll fluorescence (SIF) in vegetation: 50 years of progress,” Remote Sens Environ, vol. 231, p. 111177, Sep. 2019, doi: 10.1016/J.RSE.2019.04.030. DOI: https://doi.org/10.1016/j.rse.2019.04.030
I. Sperdouli, I. Mellidou, and M. Moustakas, “Harnessing chlorophyll fluorescence for phenotyping analysis of wild and cultivated tomato for high photochemical efficiency under water deficit for climate change resilience,” Climate, vol. 9, no. 11, Nov. 2021, doi: 10.3390/cli9110154. DOI: https://doi.org/10.3390/cli9110154
J. Yao et al., “Phenotyping of Arabidopsis drought stress response using kinetic chlorophyll fluorescence and multicolor fluorescence imaging,” Front Plant Sci, vol. 9, p. 603, May 2018, doi: 10.3389/FPLS.2018.00603/BIBTEX. DOI: https://doi.org/10.3389/fpls.2018.00603
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 EPISTEMUS

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The magazine acquires the patrimonial rights of the articles only for diffusion without any purpose of profit, without diminishing the own rights of authorship.
The authors are the legitimate owners of the intellectual property rights of their respective articles, and in such quality, by sending their texts they express their desire to collaborate with the Epistemus Magazine, published biannually by the University of Sonora.
Therefore, freely, voluntarily and free of charge, once accepted the article for publication, they give their rights to the University of Sonora for the University of Sonora to edit, publish, distribute and make available through intranets, Internet or CD said work, without any limitation of form or time, as long as it is non-profit and with the express obligation to respect and mention the credit that corresponds to the authors in any use that is made of it.
It is understood that this authorization is not an assignment or transmission of any of your economic rights in favor of the said institution. The University of Sonora guarantees the right to reproduce the contribution by any means in which you are the author, subject to the credit being granted corresponding to the original publication of the contribution in Epistemus.
Unless otherwise indicated, all the contents of the electronic edition are distributed under a license for use and Creative Commons — Attribution-NonCommercial-ShareAlike 4.0 International — (CC BY-NC-SA 4.0) You can consult here the informative version and the legal text of the license. This circumstance must be expressly stated in this way when necessary.
The names and email addresses entered in this journal will be used exclusively for the purposes established in it and will not be provided to third parties or for their use for other purposes.