Catalytic Photodecomposition of Ferrocyanide
DOI:
https://doi.org/10.36790/epistemus.v18i36.328Keywords:
Ferrocyanide, Sunlight, Decomposition, Catalyst, Oxidizing agentsAbstract
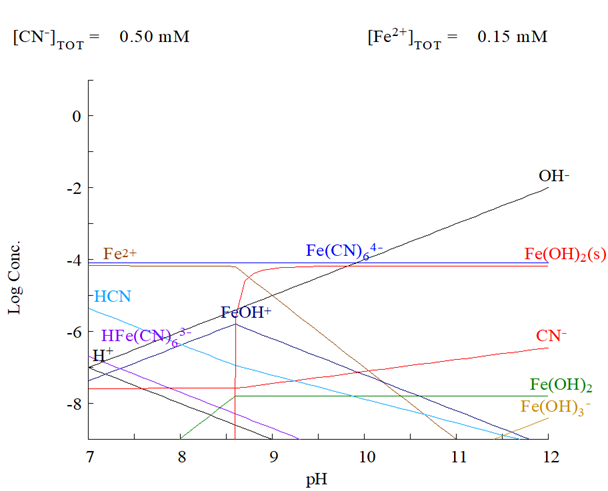
In the hydrometallurgical processes, cyanide is one of the most widely used compounds in gold extraction; however, the main problem is the generation of wastewater containing this compound and its derivatives, including cyanide-iron compounds, or better known as ferrocyanide. For the decomposition of this type of compounds there are techniques known as advanced oxidation processes, based on the formation of hydroxyl radicals. The present work focuses on the decomposition of ferrocyanide with advanced oxidation processes with solar radiation as the main agent and the use of a catalyst. Experimental tests were carried out with a synthetic solution of ferrocyanide (500 ppm) under controlled laboratory conditions. The results showed that a 62% recovery of iron in the form of precipitates was obtained in a period of one hour, while with a catalyst it reached 79%.
Downloads
References
J. A. Hernández Viveros. “Desarrollo del proceso de degradación de cianuro procedente de la lixiviación de plata a partir de oxidación con ozono cloruro de sodio”, Tesis, Universidad Autónoma Metropolitana Azcapotzalco, Ciudad de México. 2019.
B. G Pérez. ¨ Desarrollo de un nuevo método para la eliminación de cianuro de aguas residuales de mina¨, Tesis, Universidad de Oviedo, España. 2007.
B. Schulz. Introducción a la metalurgia en hidrometalurgia: Universidad de Santiago de Chile, Chile.2003, pp188-201.
S. M. K. Sadr, D. P., Saroj, J. C., Mierzwa, S. J., McGrane, G, Skouteris, R., Farmani, X., Kazos, B. M., Aumeier, s. , Kouchaki, S. K., & Ouki, “A multi expert decision support tool for the evaluation of advanced wastewater treatment trains: A novel approach to improve urban sustainability”, Environmental Sci. & Policy, vol. 90, pp. 1–10, diciembre de 2018. Accedido el 29 de mayo de 2024. [En línea]. Disponible: https://doi.org/10.1016/j.envsci.2018.09.006 DOI: https://doi.org/10.1016/j.envsci.2018.09.006
S. B. Monge, A. T. Pinto, R. S. Ribeiro, A. M. T. Silva y C. Bengoa, Manual Técnico sobre Procesos de Oxidación Avanzada aplicados al Tratamiento de Aguas Residuales Industriales. Programa Iberoam. Cienc. Tecnol. Para El Desarro., 2018. Accedido el 15 de abril de 2022. [En línea]. Disponible: https://www.researchgate.net/publication/349737485_Manual_Tecnico_sobre_Procesos_de_Oxidacion_Avanzada_aplicados_al_Tratamiento_de_Aguas_Residuales_Industriales
D. B. Miklos, C. Remy, M. Jekel, K. G. Linden, J. E. Drewes y U. Hübner, “Evaluation of advanced oxidation processes for water and wastewater treatment – A critical review”, Water Res., vol. 139, pp. 118–131, agosto de 2018. Accedido el 29 de mayo de 2024. [En línea]. Disponible: https://doi.org/10.1016/j.watres.2018.03.042 DOI: https://doi.org/10.1016/j.watres.2018.03.042
S. Ašpergěr, “Kinetics of the decomposition of potassium ferrocyanide in ultra-violet light”, Trans. Faraday Soc., vol. 48, pp. 617–624, 1952. Accedido el 29 de mayo de 2024. [En línea]. Disponible: https://doi.org/10.1039/tf9524800617 DOI: https://doi.org/10.1039/TF9524800617
M. A. Buckingham, K. Laws, J. T. Sengel y L. Aldous, “Using iron sulphate to form both n-type and p-type pseudo-thermoelectrics: Non-hazardous and ‘second life’ thermogalvanic cells”, Green Chem., vol. 22, n.º 18, pp. 6062–6074, 2020. Accedido el 30 de mayo de 2024. [En línea]. Disponible: https://doi.org/10.1039/d0gc01878c DOI: https://doi.org/10.1039/D0GC01878C
Z. Liu, J. Kou, Y. Xing y C. Sun, “Recovery of Gold from Ore with Potassium Ferrocyanide Solution under UV Light”, Minerals, vol. 11, n.º 4, p. 387, abril de 2021. Accedido el 29 de mayo de 2024. [En línea]. Disponible: https://doi.org/10.3390/min11040387 DOI: https://doi.org/10.3390/min11040387
L. D. Michard, “Hydrogen Peroxide Cyanide Destruction Plant”. 911 Metallurgist. Accedido el 21 de febrero de 2023. [En línea]. Disponible: https://www.911metallurgist.com/blog/hydrogen-peroxide-cyanide-destruction-plant
H. Amaouche et al., “Removal of cyanide in aqueous solution by oxidation with hydrogen peroxide catalyzed by copper oxide”, Water Sci. Technol., vol. 80, n.º 1, pp. 126–133, julio de 2019. Accedido el 29 de mayo de 2024. [En línea]. Disponible: https://doi.org/10.2166/wst.2019.254 DOI: https://doi.org/10.2166/wst.2019.254
Y. Wei et al., “Effectiveness and mechanism of cyanide remediation from contaminated soils using thermally activated persulfate”, Chemosphere, vol. 292, p. 133463, abril de 2022. Accedido el 17 de mayo de 2023. [En línea]. Disponible: https://doi.org/10.1016/j.chemosphere.2021.133463 DOI: https://doi.org/10.1016/j.chemosphere.2021.133463
A. Son et al., “Ti3+ self-doped TiO2 nanotube arrays revisited as Janus photoelectrodes for persulfate activation and water treatment”, Appl. Catalysis B: Environmental, vol. 315, p. 121543, octubre de 2022. Accedido el 9 de mayo de 2023. [En línea]. Disponible: https://doi.org/10.1016/j.apcatb.2022.121543 DOI: https://doi.org/10.1016/j.apcatb.2022.121543
D. F. Caicedo, I. A. S. Brum y L. A. B. Buitrago, “Photocatalytic degradation of ferricyanide as synthetic gold mining wastewater using TiO2 assisted by H2O2”, REM - Int. Eng. J., vol. 73, n.º 1, pp. 99–107, marzo de 2020. Accedido el 2 de febrero de 2023. [En línea]. Disponible: https://doi.org/10.1590/0370-44672019730042 DOI: https://doi.org/10.1590/0370-44672019730042
B. G. Pérez, “Desarrollo de un nuevo método para la eliminación de cianuro de aguas residuales de mina”, Univ. Oviedo, Oviedo, 2007.
L. A. Betancourt-Buitrago, O. E. Ossa-Echeverry, J. C. Rodriguez-Vallejo, J. M. Barraza, N. Marriaga y F. Machuca-Martínez, “Anoxic photocatalytic treatment of synthetic mining wastewater using TiO2and scavengers for complexed cyanide recovery”, Photochem. & Photobiol. Sci., vol. 18, n.º 4, pp. 853–862, 2019. Accedido el 16 de agosto de 2022. [En línea]. Disponible: https://doi.org/10.1039/c8pp00281a DOI: https://doi.org/10.1039/c8pp00281a
M. R. Al-Mamun, S. Kader, M. S. Islam y M. Z. H. Khan, “Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review”, J. Environmental Chem. Eng., vol. 7, n.º 5, p. 103248, octubre de 2019. Accedido el 29 de enero de 2023. [En línea]. Disponible: https://doi.org/10.1016/j.jece.2019.103248 DOI: https://doi.org/10.1016/j.jece.2019.103248
B. Tsybikova, “Solar energy efficient - AOP process for treatment of cyanide in mining effluents”, IOP Conf. Ser.: Mater. Sci. Eng., vol. 962, p. 042079, noviembre de 2020. Accedido el 12 de mayo de 2022. [En línea]. Disponible: https://doi.org/10.1088/1757-899x/962/4/042079 DOI: https://doi.org/10.1088/1757-899X/962/4/042079
P. Wang, C. Qi, P. Wen, L. Hao, X. Xu y S. Agathopoulos, “Synthesis of Si, N co-Doped Nano-Sized TiO2 with High Thermal Stability and Photocatalytic Activity by Mechanochemical Method”, Nanomaterials, vol. 8, n.º 5, p. 294, mayo de 2018. [En línea]. Disponible: https://doi.org/10.3390/nano8050294 DOI: https://doi.org/10.3390/nano8050294
A. K. H. Bashir et al., “Biosynthesis of NiO nanoparticles for photodegradation of free cyanide solutions under ultraviolet light”, J. Phys. Chemistry Solids, vol. 134, pp. 133–140, noviembre de 2019. [En línea]. Disponible: https://doi.org/10.1016/j.jpcs.2019.05.048 DOI: https://doi.org/10.1016/j.jpcs.2019.05.048
S. F. Castilla-Acevedo, L. A. Betancourt-Buitrago, D. D. Dionysiou y F. Machuca-Martínez, “Ultraviolet light-mediated activation of persulfate for the degradation of cobalt cyanocomplexes”, J. Hazardous Mater., vol. 392, p. 122389, junio de 2020. [En línea]. Disponible: https://doi.org/10.1016/j.jhazmat.2020.122389 DOI: https://doi.org/10.1016/j.jhazmat.2020.122389
N. Ghasemi y S. Rohani, “Optimization of cyanide removal from wastewaters using a new nano-adsorbent containing ZnO nanoparticles and MOF/Cu and evaluating its efficacy and prediction of experimental results with artificial neural networks”, J. Mol. Liquids, vol. 285, pp. 252–269, julio de 2019.[En línea]. Disponible: https://doi.org/10.1016/j.molliq.2019.04.085 DOI: https://doi.org/10.1016/j.molliq.2019.04.085
F. Wang, H. Zhang, X. Xiong, K. Tian, B., Gao, Y., Sun, & T. B. Yu, “Application of heterogeneous catalytic ozonation for refractory organics in wastewater”, Catalysts, vol. 9, n.º 3, p. 241, marzo de 2019. [En línea]. Disponible: https://doi.org/10.3390/catal9030241 DOI: https://doi.org/10.3390/catal9030241
L. Yan, J. Bing y H. Wu, “The behavior of ozone on different iron oxides surface sites in water”, Scientific Rep., vol. 9, n.º 1, octubre de 2019. [En línea]. Disponible: https://doi.org/10.1038/s41598-019-50910-w DOI: https://doi.org/10.1038/s41598-019-50910-w
V. Garzón-Cucaita y J. G. Carriazo, “Óxidos de hierro como catalizadores de procesos tipo Fenton con potencial aplicación en tecnologías de remoción de contaminantes”, TecnoLógicas, vol. 25, n.º 55, noviembre de 2022, art. n.º e2393. [En línea]. Disponible: https://doi.org/10.22430/22565337.2393 DOI: https://doi.org/10.22430/22565337.2393
M. R. Garza Román, F. R. Carrillo Pedroza, M. d. J. Soria Aguilar y N. G. Picazo Rodriguez, “Descomposición de cianuro usando ozono y óxidos de hierro”, Epistemus, vol. 15, n.º 31, abril de 2022.[En línea]. Disponible: https://doi.org/10.36790/epistemus.v15i31.202 DOI: https://doi.org/10.36790/epistemus.v15i31.202
M. R. Garza-Román, V. D. Treviño-Rodriguez, M. Soria-Aguilar, F. R. Carrillo-Pedroza, , N. Picazo-Rodriguez, M. A. Sánchez. ¨Remoción de ferrocianuro con óxidos de hierro, persulfato de sodio e irradiación solar¨, 2022. https://repositorioinstitucional.uaslp.mx/xmlui/handle/i/8025
D. R. Eaton y M. Pankratz, “The reaction of the hexacyanoferrate(III) ion with hydrogen peroxide”, Can. J. Chemistry, vol. 63, n.º 4, pp. 793–797, abril de 1985. [En línea]. Disponible: https://doi.org/10.1139/v85-131 DOI: https://doi.org/10.1139/v85-131
J. H. Sánchez-Moreno, ¨Impacto del Óxido de Zinc (ZnO) y Dióxido de Titanio (TiO2) como fotocatalizadores en la degradación oxidativa del índigo, en efluentes generados por la Industria Textilera¨. Polo del Conocimiento, pp. 1249-1269, feb. 2022. ISSN 2550-682X
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 EPISTEMUS

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The magazine acquires the patrimonial rights of the articles only for diffusion without any purpose of profit, without diminishing the own rights of authorship.
The authors are the legitimate owners of the intellectual property rights of their respective articles, and in such quality, by sending their texts they express their desire to collaborate with the Epistemus Magazine, published biannually by the University of Sonora.
Therefore, freely, voluntarily and free of charge, once accepted the article for publication, they give their rights to the University of Sonora for the University of Sonora to edit, publish, distribute and make available through intranets, Internet or CD said work, without any limitation of form or time, as long as it is non-profit and with the express obligation to respect and mention the credit that corresponds to the authors in any use that is made of it.
It is understood that this authorization is not an assignment or transmission of any of your economic rights in favor of the said institution. The University of Sonora guarantees the right to reproduce the contribution by any means in which you are the author, subject to the credit being granted corresponding to the original publication of the contribution in Epistemus.
Unless otherwise indicated, all the contents of the electronic edition are distributed under a license for use and Creative Commons — Attribution-NonCommercial-ShareAlike 4.0 International — (CC BY-NC-SA 4.0) You can consult here the informative version and the legal text of the license. This circumstance must be expressly stated in this way when necessary.
The names and email addresses entered in this journal will be used exclusively for the purposes established in it and will not be provided to third parties or for their use for other purposes.