Lead Leaching with Citrate from a Galena Mineral, pH Effect
DOI:
https://doi.org/10.36790/epistemus.v17i34.273Keywords:
Lead, Leaching, Citrate, AlkalineAbstract
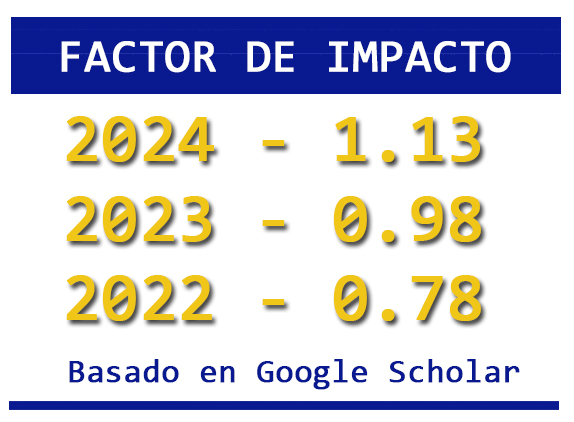
The recovery of metals from minerals through alternative processes is appropriate for the reduction of pollutants produced in foundries and traditional operations; the leaching of metals with the use of chemical reagents at low concentrations allows the reuse of the solutions, as well as the reduction of problems in the recycling of the solutions. Leaching with organic reagents at room temperature reduces the issues in the generation of gases and consumption of reagents, which is why it turns out to be an option for obtaining metals from minerals. This work shows an alternative for lead recovery by leaching a galena mineral using sodium citrate in a concentration of 0.2 M at room temperature. The effect of pH in acid, neutral, and alkaline mediums is evaluated, where recovery of 99.9% of lead in alkaline media is achieved.
Downloads
References
David O'Connor, Deyi Hou, Jing Ye, Yunhui Zhang, Yong Sik Ok, Yinan Song, Frederic Coulon, Tianyue Peng, Li Tian, (2018). "Lead-based paint remains a major public health concern: A critical review of global production, trade, use, exposure, health risk, and implications." Environment International 121: 85-101. https://doi.org/10.1016/j.envint.2018.08.052. DOI: https://doi.org/10.1016/j.envint.2018.08.052
United States Geological Survey, USGS (2021). Mineral Commodity Summary. Series definitions. USGS Publications Warehouse.
Chuh-Shun Chen, Yu-Jen Shih, Yao-Hui Huang (2016). Recovery of lead from smelting fly ash of waste lead-acid battery by leaching and electrowinning. Waste Management, 52, 212-220. doi:https://doi.org/10.1016/j.wasman.2016.03.056 DOI: https://doi.org/10.1016/j.wasman.2016.03.056
Y.S. Rammah, K.A. Mahmoud, M.I. Sayyed, F.I. El-Agawany, R. El-Mallawany (2020). "Novel vanadyl lead-phosphate glasses: P2O5–PbO–ZnONa2O–V2O5: Synthesis, optical, physical and gamma photon attenuation properties." Journal of Non-Crystalline Solids 534: 119944. https://doi.org/10.1016/j.jnoncrysol.2020.119944 DOI: https://doi.org/10.1016/j.jnoncrysol.2020.119944
John, J. J., De Houwer, V., Van Mechelen, D., & Van Gerven, T. (2020). Effect of ultrasound on leaching of lead from landfilled metallurgical residues. Ultrasonics Sonochemistry, 69, 105239. doi:https://doi.org/10.1016/j.ultsonch.2020.105239 DOI: https://doi.org/10.1016/j.ultsonch.2020.105239
Hou, Z., Peng, C., Guangyuan, M., Zhang, X., Zhang, L., & Lu, Z. (2022). Lead leaching behaviors during panel-funnel glass waste separation from cathode-ray tube glass using thermosol, acid etching or mechanical cutting methods. Journal of Saudi Chemical Society, 26(6), 101557. doi:https://doi.org/10.1016/j.jscs.2022.101557 DOI: https://doi.org/10.1016/j.jscs.2022.101557
Zárate-Gutiérrez, R. and G. T. Lapidus (2014). "Anglesite (PbSO4) leaching in citrate solutions." Hydrometallurgy 144-145: 124-128. https://doi.org/10.1016/j.hydromet.2014.02.003 DOI: https://doi.org/10.1016/j.hydromet.2014.02.003
Xie, H., Xiao, X., Guo, Z., & Li, S. (2022). One-stage ultrasonic-assisted calcium chloride leaching of lead from zinc leaching residue. Chemical Engineering and Processing - Process Intensification, 176, 108941. doi:https://doi.org/10.1016/j.cep.2022.108941 DOI: https://doi.org/10.1016/j.cep.2022.108941
Xinfeng Zhu 1, Xiong He, Jiakuan Yang, Linxia Gao, Jianwen Liu, Danni Yang, Xiaojuan Sun, Wei Zhang, Qin Wang, R Vasant Kumar.(2013) "Leaching of spent lead acid battery paste components by sodium citrate and acetic acid," Journal of Hazardous Materials, vol. 250-251, pp. 387-396, 2013/04/15/ 2013. DOI: https://doi.org/10.1016/j.jhazmat.2013.02.018
G. Han, P. Wang, C. Zhu, B. Liu, S. Su, and Y. Huang, "Synchronous extraction of Mn, Pb, Sn, and Se from hazardous electrolytic manganese anode slime (EMAS) via sulfidation transformation and leaching: Rapid separation and sulfidation mechanism," Minerals Engineering, vol. 184, p. 107657, https://doi.org/10.1016/j.mineng.2022.107657 DOI: https://doi.org/10.1016/j.mineng.2022.107657
NIST (2004). Critically Selected Stability Constants of Metal Complexes. Version 8.0.
Puigdomenech, I. (2004). Make equilibrium diagrams using sophisticated algorithms (MEDUSA), inorganic chemistry. Stockholm, Sweden

Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 EPISTEMUS

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The magazine acquires the patrimonial rights of the articles only for diffusion without any purpose of profit, without diminishing the own rights of authorship.
The authors are the legitimate owners of the intellectual property rights of their respective articles, and in such quality, by sending their texts they express their desire to collaborate with the Epistemus Magazine, published biannually by the University of Sonora.
Therefore, freely, voluntarily and free of charge, once accepted the article for publication, they give their rights to the University of Sonora for the University of Sonora to edit, publish, distribute and make available through intranets, Internet or CD said work, without any limitation of form or time, as long as it is non-profit and with the express obligation to respect and mention the credit that corresponds to the authors in any use that is made of it.
It is understood that this authorization is not an assignment or transmission of any of your economic rights in favor of the said institution. The University of Sonora guarantees the right to reproduce the contribution by any means in which you are the author, subject to the credit being granted corresponding to the original publication of the contribution in Epistemus.
Unless otherwise indicated, all the contents of the electronic edition are distributed under a license for use and Creative Commons — Attribution-NonCommercial-ShareAlike 4.0 International — (CC BY-NC-SA 4.0) You can consult here the informative version and the legal text of the license. This circumstance must be expressly stated in this way when necessary.
The names and email addresses entered in this journal will be used exclusively for the purposes established in it and will not be provided to third parties or for their use for other purposes.