Scorpions from Sonora, Mexico and their Biotechnological Potential
DOI:
https://doi.org/10.36790/epistemus.v19i38.408Keywords:
scorpions, venoms, pharmacology, medicine, biopharmaceuticalsAbstract
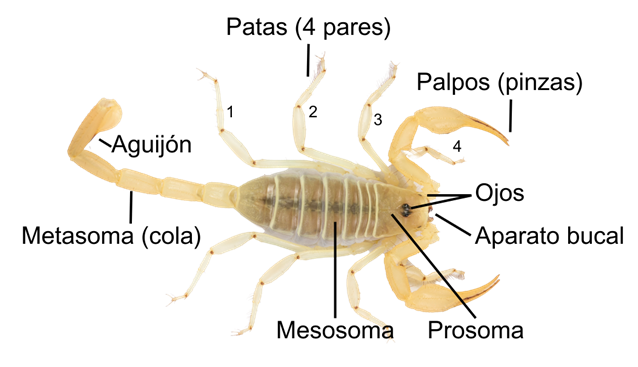
Scorpions are arachnids, easily recognizable by the presence of a tail that ends in a stinger as well as of pincers like those of crustaceans. Mexico ranks first worldwide in terms of the richness of these animals, with more than 300 species described. On the other hand, in the state of Sonora, more than 40 species have been reported and only 4 are considered of medical toxicological importance. In that matter, studies have revealed that the toxins present in scorpion venoms mainly affect ion channels, some of which are extremely powerful and may endanger human life. Additionally, it has been reported that some of these toxins have biotechnological potential for the development of biopharmaceuticals and diagnostic agents, making them a rich source of bioactive molecules. In this article we discuss the general characteristics of these animals, their venoms, and their potential in the areas of biomedical research.
Downloads
References
C. E. Santibáñez-López, O. F. Francke, C. Ureta, and L. D. Possani, “Scorpions from Mexico: From Species Diversity to Venom Complexity,” Toxins (Basel), vol. 8, no. 1, Dec. 2015, doi: 10.3390/toxins8010002. DOI: https://doi.org/10.3390/toxins8010002
Y. Liu et al., “Differential fluorescence features and recovery speeds of different scorpion exoskeleton parts during the molting process,” Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, vol. 316, p. 124309, Aug. 2024, doi: 10.1016/j.saa.2024.124309. DOI: https://doi.org/10.1016/j.saa.2024.124309
C. Shimwell, L. Atkinson, M. R. Graham, and B. Murdoch, “A first molecular characterization of the scorpion telson microbiota of Hadrurus arizonensis and Smeringurus mesaensis,” PLOS ONE, vol. 18, no. 1, p. e0277303, ene 2023, doi: 10.1371/journal.pone.0277303. DOI: https://doi.org/10.1371/journal.pone.0277303
J. Ponce-Saavedra, O. F. F. B, A. F. Quijano-Ravell, and R. C. Santillán, “ALACRANES (ARACHNIDA: SCORPIONES) DE IMPORTANCIA PARA LA SALUD PÚBLICA EN MÉXICO,” Folia Entomológica
Mexicana (nueva serie), vol. 2, no. 3, Art. no. 3, Dec. 2016.
J. I. Cid-Uribe, J. I. Veytia-Bucheli, T. Romero-Gutierrez, E. Ortiz, and L. D. Possani, “Scorpion venomics: a 2019 overview,” Expert Review of Proteomics, vol. 17, no. 1, pp. 67–83, Jan. 2020, doi: 10.1080/14789450.2020.1705158. DOI: https://doi.org/10.1080/14789450.2020.1705158
“The Scorpion Files - Main Page.” Accessed: Oct. 14, 2024. [Online]. Available: https://www.ntnu.no/ub/scorpion-files/
H. H. Villa-Corella, H. Silva-Kurumiya, D. Barrales-Alcalá, T. R. V. Devender, and O. F. Francke, “Una especie nueva de Diplocentrus Peters, 1861 (Scorpiones: Diplocentridae) del estado de Sonora, México,” ACTA ZOOLÓGICA MEXICANA (N.S.), pp. 1–14, Mar. 2023, doi: 10.21829/azm.2023.3912277. DOI: https://doi.org/10.21829/azm.2023.3912277
J. Ponce-Saavedra et al., “The Fauna of Arachnids in the Anthropocene of Mexico,” in Mexican Fauna in the Anthropocene, R. W. Jones, C. P. Ornelas-García, R. Pineda-López, and F. Álvarez, Eds., Cham: Springer International Publishing, 2023, pp. 17–46. doi: 10.1007/978-3-031-17277-9_2. DOI: https://doi.org/10.1007/978-3-031-17277-9_2
L. Riaño-Umbarila et al., “Toxic Peptides from the Mexican Scorpion Centruroides villegasi: Chemical Structure and Evaluation of Recognition by Human Single-Chain Antibodies,” Toxins, vol. 16, no. 7, Art. no. 7, Jul. 2024, doi: 10.3390/toxins16070301. DOI: https://doi.org/10.3390/toxins16070301
“iNaturalist Mexico,” iNaturalist Mexico. Accessed: Jan. 18, 2025. [Online]. Available: https://mexico.inaturalist.org/
S. de Salud, “BoletínEpidemiológico Sistema Nacional de Vigilancia Epidemiológica Sistema Único de Información,” gob.mx. Accessed: Oct. 09, 2024. [Online]. Available: http://www.gob.mx/salud/documentos/boletinepidemiologico-sistema-nacional-de-vigilancia-epidemiologica-sistema-unico-de-informacion-355523
D. D. Gummin et al., “2022 Annual Report of the National Poison Data System® (NPDS) from America’s Poison Centers®: 40th Annual Report,” Clinical Toxicology, vol. 61, no. 10, pp. 717–939, Oct. 2023, doi: 10.1080/15563650.2023.2268981. DOI: https://doi.org/10.1080/15563650.2023.2268981
“ANASCORP® [centruroides (scorpion) immune F(ab’)? (equine) injection] |.” Accessed: Oct. 09, 2024. [Online]. Available: https://www.anascorp-us.com/
J. Jimenez-Canale et al., “Exploring the protein profile and biological activity of Crotalus molossus venom against E. coli, P. aeruginosa and S. aureus bacteria and T47D breast carcinoma cells,” Toxicon, vol. 249, p. 108036, Oct. 2024, doi: 10.1016/j.toxicon.2024.108036. DOI: https://doi.org/10.1016/j.toxicon.2024.108036
R. L. Langley, “A Review of Venomous Animal Bites and Stings in Pregnant Patients,” Wilderness & Environmental Medicine, vol. 15, no. 3, pp. 207–215, Sep. 2004, doi: 10.1580/1080-6032(2004)15[207:AROVAB]2.0.CO;2. DOI: https://doi.org/10.1580/1080-6032(2004)15[207:AROVAB]2.0.CO;2
G. Murillo-Godínez, “Picadura de alacrán y alacranismo,” Med Int Mex, vol. 36, no. 5, pp. 696–712, Oct. 2020.
T. Fischer and R. Riedl, “Paracelsus’ legacy in the faunal realm: Drugs deriving from animal toxins,” Drug Discovery Today, vol. 27, no. 2, pp. 567–575, Feb. 2022, doi: 10.1016/j.drudis.2021.10.003. DOI: https://doi.org/10.1016/j.drudis.2021.10.003
R. Dingman and S. V. Balu-Iyer, “Immunogenicity of Protein Pharmaceuticals,” Journal of Pharmaceutical Sciences, vol. 108, no. 5, pp. 1637–1654, May 2019, doi: 10.1016/j.xphs.2018.12.014. DOI: https://doi.org/10.1016/j.xphs.2018.12.014
Jimenez Canale, Jorge, E. F. Velázquez-Contreras, and Sarabia Sainz, Jose Andrei, “El potencial farmacológico de venenos de serpientes de Sonora, México | EPISTEMUS,” EPISTEMUS, vol. 16, no. 33, Nov. 2022, doi: https://doi.org/10.36790/epistemus.v16i33.226.
Mohammadreza Moradi, R. Solgi, B. Vazirianzadeh, H. Tanzadehpanah, and M. Saidijam, “SCORPION VENOM AND ITS COMPONENTS AS NEW PHARMACEUTICAL APPROACH TO CANCER TREATMENT, A SYSTEMATIC REVIEW,” IJPSR, vol. 9, no. 7.
Y. ZHANG, “Why do we study animal toxins?,” Dongwuxue Yanjiu, vol. 36, no. 4, pp. 183–222, Jul. 2015, doi: 10.13918/j.issn.2095-8137.2015.4.183.
J. Jimenez Canale, E. F. Velázquez-Contreras, and Sarabia Sainz, Jose Andrei, “El potencial farmacológico de venenos de serpientes de Sonora, México | EPISTEMUS,” EPISTEMUS, vol. 16, no. 33, 2022B, doi: https://doi.org/10.36790/epistemus.v16i33.226. DOI: https://doi.org/10.36790/epistemus.v16i33.226
A. N. de Oliveira, A. M. Soares, and S. L. Da Silva, “Why to Study Peptides from Venomous and Poisonous Animals?,” Int J Pept Res Ther, vol. 29, no. 5, p. 76, Jul. 2023, doi: 10.1007/s10989-023-10543-0. DOI: https://doi.org/10.1007/s10989-023-10543-0
F. J. Vonk et al., “A non-lethal method for studying scorpion venom gland transcriptomes, with a review of potentially suitable taxa to which it can be applied,” PLOS ONE, vol. 16, no. 11, p. e0258712, Nov. 2021, doi: 10.1371/journal.pone.0258712. DOI: https://doi.org/10.1371/journal.pone.0258712
Z.-F. Chai et al., “Chinese-scorpion (Buthus martensi Karsch) toxin BmK αIV, a novel modulator of sodium channels: from genomic organization to functional analysis,” Biochemical Journal, vol. 399, no. 3, pp. 445–453, Oct. 2006, doi: 10.1042/BJ20060035. DOI: https://doi.org/10.1042/BJ20060035
P. Cao et al., “Expression and purification of an antitumor-analgesic peptide from the venom of Mesobuthus martensii Karsch by small ubiquitin–related modifier fusion in Escherichia coli,” Biotechnology Progress, vol. 26, no. 5, pp. 1240–1244, 2010, doi: 10.1002/btpr.433. DOI: https://doi.org/10.1002/btpr.433
A. Daniele-Silva et al., “Stigmurin and TsAP-2 from Tityus stigmurus scorpion venom: Assessment of structure and therapeutic potential in experimental sepsis,” Toxicon, vol. 121, pp. 10–21, Oct. 2016, doi: 10.1016/j.toxicon.2016.08.016. DOI: https://doi.org/10.1016/j.toxicon.2016.08.016
A. Torres-Larios, G. B. Gurrola, F. Z. Zamudio, and L. D. Possani, “Hadrurin, a new antimicrobial peptide from the venom of the scorpion Hadrurus aztecus,” European Journal of Biochemistry, vol. 267, no. 16, pp. 5023–5031, 2000, doi: 10.1046/j.1432-1327.2000.01556.x. DOI: https://doi.org/10.1046/j.1432-1327.2000.01556.x
J. Deshane, C. C. Garner, and H. Sontheimer, “Chlorotoxin Inhibits Glioma Cell Invasion via Matrix Metalloproteinase-2,” J. Biol. Chem., vol. 278, no. 6, pp. 4135–4144, Feb. 2003, doi: 10.1074/jbc.M205662200. DOI: https://doi.org/10.1074/jbc.M205662200
S. Nasr et al., “Scorpion Venom as a Source of Antimicrobial Peptides: Overview of Biomolecule Separation, Analysis and Characterization Methods,” Antibiotics, vol. 12, no. 9, Art. no. 9, Sep. 2023, doi: 10.3390/antibiotics12091380. DOI: https://doi.org/10.3390/antibiotics12091380
E. N. Carcamo-Noriega et al., “1,4-Benzoquinone antimicrobial agents against Staphylococcus aureus and Mycobacterium tuberculosis derived from scorpion venom,” Proc Natl Acad Sci USA, vol. 116, no. 26, pp. 12642–12647, Jun. 2019, doi: 10.1073/pnas.1812334116. DOI: https://doi.org/10.1073/pnas.1812334116
S. A. Lyons, J. O’Neal, and H. Sontheimer, “Chlorotoxin, a scorpion-derived peptide, specifically binds to gliomas and tumors of neuroectodermal origin,” Glia, vol. 39, no. 2, pp. 162–173, 2002, doi: 10.1002/glia.10083. DOI: https://doi.org/10.1002/glia.10083
M. Nosouhian, A. A. Rastegari, K. Shahanipour, A. M. Ahadi, and M. S. Sajjadieh, “Anticancer potentiality of Hottentotta saulcyi scorpion curd venom against breast cancer: an in vitro and in vivo study,” Sci Rep, vol. 14, no. 1, p. 24607, Oct. 2024, doi: 10.1038/s41598-024-75183-w. DOI: https://doi.org/10.1038/s41598-024-75183-w
S. Tobassum, H. M. Tahir, M. Arshad, M. T. Zahid, S. Ali, and M. M. Ahsan, “Nature and applications of scorpion venom: an overview,” Toxin Reviews, vol. 39, no. 3, pp. 214–225, Jul. 2020, doi: 10.1080/15569543.2018.1530681. DOI: https://doi.org/10.1080/15569543.2018.1530681
M. Najafian, A. Ghorbani, M. Zargar, M. Baradaran, and N. Baradaran, “Scorpion stings in pregnancy: an analysis of outcomes in 66 envenomed pregnant patients in Iran,” J. Venom. Anim. Toxins incl. Trop. Dis, vol. 26, p. e20190039, Apr. 2020, doi: 10.1590/1678-9199-JVATITD-2019-0039. DOI: https://doi.org/10.1590/1678-9199-jvatitd-2019-0039
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 EPISTEMUS

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The magazine acquires the patrimonial rights of the articles only for diffusion without any purpose of profit, without diminishing the own rights of authorship.
The authors are the legitimate owners of the intellectual property rights of their respective articles, and in such quality, by sending their texts they express their desire to collaborate with the Epistemus Magazine, published biannually by the University of Sonora.
Therefore, freely, voluntarily and free of charge, once accepted the article for publication, they give their rights to the University of Sonora for the University of Sonora to edit, publish, distribute and make available through intranets, Internet or CD said work, without any limitation of form or time, as long as it is non-profit and with the express obligation to respect and mention the credit that corresponds to the authors in any use that is made of it.
It is understood that this authorization is not an assignment or transmission of any of your economic rights in favor of the said institution. The University of Sonora guarantees the right to reproduce the contribution by any means in which you are the author, subject to the credit being granted corresponding to the original publication of the contribution in Epistemus.
Unless otherwise indicated, all the contents of the electronic edition are distributed under a license for use and Creative Commons — Attribution-NonCommercial-ShareAlike 4.0 International — (CC BY-NC-SA 4.0) You can consult here the informative version and the legal text of the license. This circumstance must be expressly stated in this way when necessary.
The names and email addresses entered in this journal will be used exclusively for the purposes established in it and will not be provided to third parties or for their use for other purposes.