La Hidrometalurgia del zinc: una solución sostenible para concentrados minerales y residuos mineros
DOI:
https://doi.org/10.36790/epistemus.v19i38.320Palabras clave:
Zinc, Economía circular, Hiidrometalurgia, Sostenibilidad, Residuos minerosResumen
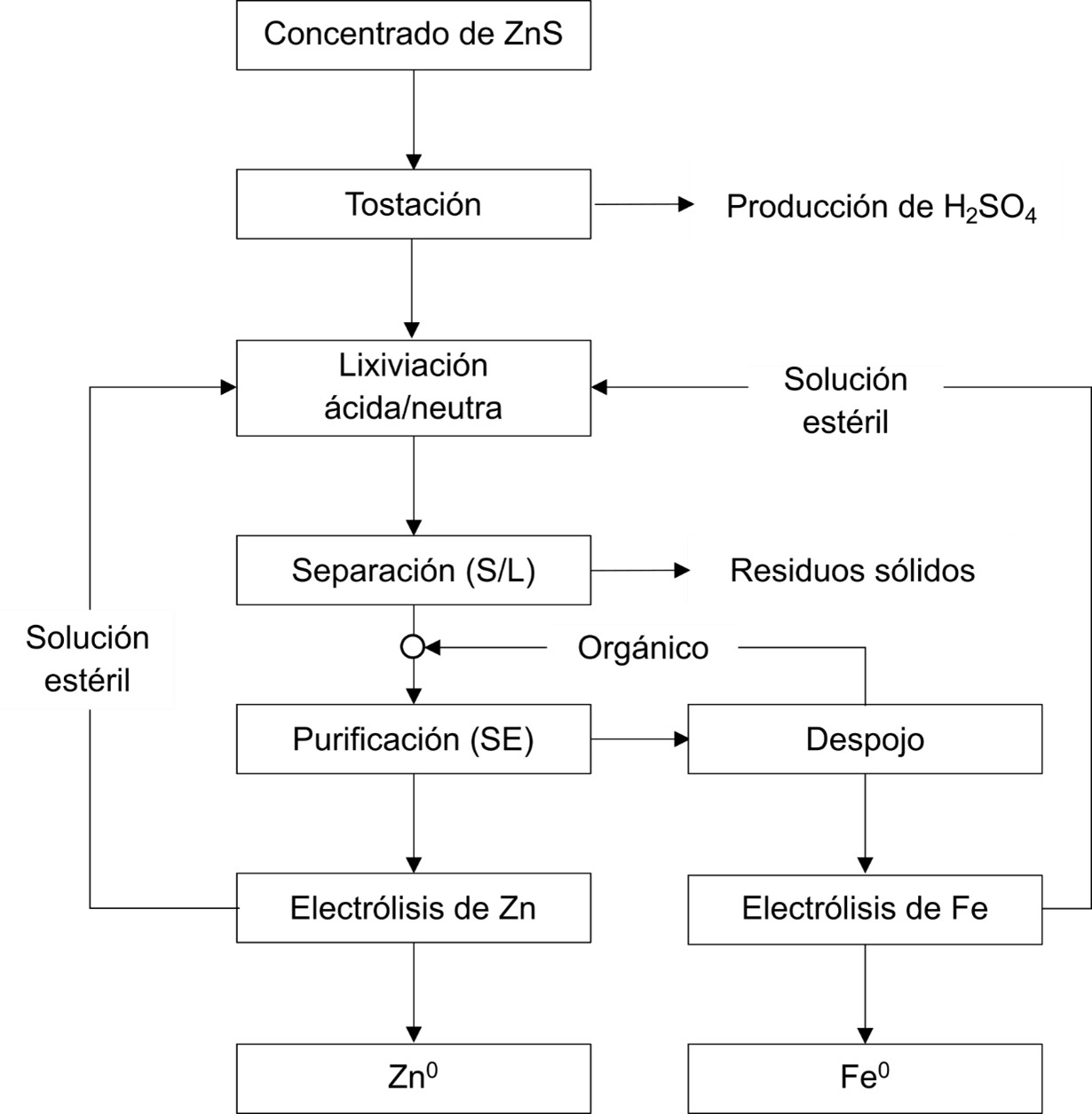
El zinc es un metal con diversas aplicaciones industriales que se puede obtener por la vía hidrometalúrgica y pirometalúrgica. En esta revisión se abordan los avances frente a la producción sostenible de este metal. Este enfoque implica la adopción de prácticas y procesos que minimicen el impacto ambiental y fomenten el uso responsable de los recursos. Asimismo, se exploran las principales aplicaciones del zinc y la demanda proyectada a futuro. Además, se revisa la obtención de zinc a partir de fuentes secundarias como pilas usadas, chatarra electrónica, aleaciones de zinc, entre otras. Dichos procesos introducen el concepto de minería urbana, el cual promueve la transición hacia una economía circular. El reciclaje representa una actividad económicamente viable y contribuye a disminuir el volumen de residuos enviados a las presas de jales.
Descargas
Citas
M. Pais and P. Rao, “An Up-to-Date Review on Industrially Significant Inhibitors for Corrosion Control of Zinc,” J. Bio. Tribo. Corrosion, vol. 7, no. 3, 2021, DOI: https://doi:10.1007/s40735-021-00556-x. DOI: https://doi.org/10.1007/s40735-021-00556-x
H. Kania and M. Saternus, “Evaluation and Current State of Primary and Secondary Zinc Production—A Review”. Appl. Sci., vol. 13, no. 3, p. 2003, 2023. DOI: https://doi:10.3390/app13032003. DOI: https://doi.org/10.3390/app13032003
“Home" | ZINC. International Zinc Association. Home | ZINC. International Zinc Association. Accessed: June 7, 2023. [Online]. Available: https://www.zinc.org/
R.J. Sinclair. The Extractive Metallurgy of Zinc. Spectrum Series. The Australasian Institute of Mining and Metallurgy, 2005.
M. K. Jha, V. Kumar and R. J. Singh, “Review of hydrometallurgical recovery of zinc from industrial wastes”, Resources, Conservation Recycling, vol. 33, no. 1, pp. 1–22, 2001. DOI: https://doi:10.1016/s0921-3449(00)00095-1. DOI: https://doi.org/10.1016/S0921-3449(00)00095-1
E. C. I. Hammerbeck and A. T. M. Mehliss. In: Ore Deposits of the Republic of South Africa (eds.), pp. 461. Pretoria, South Africa: Department of Mining and the Geological Survey of South Africa, The Government Printer, 1976.
A. Maihatchi Ahamed, M. N. Pons, Q. Ricoux, S. Issa, F. Goettmann and F. Lapicque, “New pathway for utilization of jarosite, an industrial waste of zinc hydrometallurgy”, Minerals Eng., vol. 170, p. 107030, 2021. DOI: https://doi:10.1016/j.mineng.2021.107030. DOI: https://doi.org/10.1016/j.mineng.2021.107030
V. Masloboev, S. Seleznev, A. Svetlov and D. Makarov, “Hydrometallurgical Processing of Low-Grade Sulfide Ore and Mine Waste in the Arctic Regions: Perspectives and Challenges”, Minerals, vol. 8, no. 10, p. 436, 2018. DOI: https://doi:10.3390/min8100436. DOI: https://doi.org/10.3390/min8100436
M. Shamsuddin, Physical Chemistry of Metallurgical Processes, Second Edition. Cham: Springer International Publishing, 2021. DOI: https://doi:10.1007/978-3-030-58069-8. DOI: https://doi.org/10.1007/978-3-030-58069-8
B. Sun, C. Yang, H. Zhu, Y. Li and W. Gui, “Modeling, optimization, and control of solution purification process in zinc hydrometallurgy”, IEEE/CAA J. Automatica Sinica, vol. 5, no. 2, pp. 564–576, 2018. DOI: https://doi:10.1109/jas.2017.7510844. DOI: https://doi.org/10.1109/JAS.2017.7510844
A. B. Pérez, A. Ballester and L. F Verdeja. Metalurgia extractiva, vol. II. Síntesis, 2000.
F. Habashi. Handbook of Extractive Metallurgy, vol. II and III. Wiley – VCH, 1997.
L. G. Atanasova, “Exergy analysis of the process of regeneration of spent sulphuric acid by WSA technology”, Int. J. Exergy, vol. 24, no. 1, p. 57, 2017. DOI: https://doi:10.1504/ijex.2017.086860. DOI: https://doi.org/10.1504/IJEX.2017.086860
M. R. Zaker, C. Fauteux-Lefebvre and J. Thibault, “Modelling and Multi-Objective Optimization of the Sulphur Dioxide Oxidation Process”, Processes, vol. 9, no. 6, p. 1072, 2021. DOI: https://doi:10.3390/pr9061072. DOI: https://doi.org/10.3390/pr9061072
“Pollution and Pollution Prevention”, Handbook of Pollution Prevention and Cleaner Production: Best Practices in the Agrochemical Industry. Elsevier, 2011, pp. 25–79. DOI: https://doi:10.1016/b978-1-4377-7825-0.00002-9. DOI: https://doi.org/10.1016/B978-1-4377-7825-0.00002-9
J. E. Dutrizac, “The Behaviour of the Lanthanide Elements During Jarosite Precipitation”, Electrometallurgy and Environmental Hydrometallurgy. Hoboken, NJ, USA: John Wiley & Sons, Inc., 2013, pp. 1755–1771. DOI: https://doi:10.1002/9781118804407.ch51. DOI: https://doi.org/10.1002/9781118804407.ch51
X. Zhang, J. Tian, H. Han, W. Sun, Y. Hu, T. L. Wang, Y. Yang, X. Cao and H. Tang, “Arsenic removal from arsenic-containing copper and cobalt slag using alkaline leaching technology and MgNH4AsO4 precipitation”, Separation Purification Technol., vol. 238, p. 116422, May 2020. DOI: https://doi:10.1016/j.seppur.2019.116422. DOI: https://doi.org/10.1016/j.seppur.2019.116422
F. Cardarelli, Materials Handbook. Cham: Springer International Publishing, 2018. DOI: https://doi:10.1007/978-3-319-38925-7. DOI: https://doi.org/10.1007/978-3-319-38925-7
L. Hoeber and S. Steinlechner, “A comprehensive review of processing strategies for iron precipitation residues from zinc hydrometallurgy”, Cleaner Eng. Techno., vol. 4, p. 100214, Oct. 2021. DOI: https://doi:10.1016/j.clet.2021.100214. DOI: https://doi.org/10.1016/j.clet.2021.100214
W. Wang, T. Yuan, R. Li, X. Zhu, H. Li, W. Lin, L. Li and D. Zheng, “Electrochemical corrosion behaviors of Pb-Ag anodes by electric current pulse assisted casting”, J. Electroanalytical Chemistry, vol. 847, p. 113250, Aug. 2019. DOI: https://doi:10.1016/j.jelechem.2019.113250. DOI: https://doi.org/10.1016/j.jelechem.2019.113250
A. C. Scott, R. M. Pitblado, G. W. Barton and A. R. Ault, “Experimental determination of the factors affecting zinc electrowinning efficiency”, J. Appl. Electrochemistry, vol. 18, no. 1, pp. 120–127, Jan. 1988. DOI: https://doi:10.1007/bf01016215. DOI: https://doi.org/10.1007/BF01016215
N. Sorour, W. Zhang, G. Gabra, E. Ghali and G. Houlachi, “Electrochemical studies of ionic liquid additives during the zinc electrowinning process”, Hydrometallurgy, vol. 157, pp. 261–269, Oct. 2015. DOI: https://doi:10.1016/j.hydromet.2015.09.003. DOI: https://doi.org/10.1016/j.hydromet.2015.09.003
G. T. Lapidus, “Minimizing the Hydro in Hydrometallurgy”, The Minerals, Metals & Materials Series. Cham: Springer International Publishing, 2018, pp. 1193–1201. DOI: https://doi:10.1007/978-3-319-95022-8_96. DOI: https://doi.org/10.1007/978-3-319-95022-8_96
M. Nicol, N. Welham and G. Senanayake, “Solvent extraction”, Hydrometallurgy. Elsevier, 2022, pp. 117–170. DOI: https://doi:10.1016/b978-0-323-99214-5.00007-3. DOI: https://doi.org/10.1016/B978-0-323-99214-5.00007-3
F. Khanramaki, A.R. Keshtkar. “Optimization of thorium solvent extraction process from feed solution with Cyanex 272 by response surface methodology (RSM)”. Sci Rep 14, 2024. DOI: https://doi.org/10.1038/s41598-024-66091-0 DOI: https://doi.org/10.1038/s41598-024-66091-0
P. Tahmasebizadeh, S. Javanshir, “Solvent extraction of zinc from a bioleaching solution by modification of D2EHPA: optimization and thermodynamic studies”. JME 12(1), 2021, pp. 253–269.
A. Sokolov, D. Valeev, A. Kasikov. “Solvent Extraction of Iron(III) from Al Chloride Solution of Bauxite HCl Leaching by Mixture of Aliphatic Alcohol and Ketone”. Metals, vol 11, no. 2, 2021. DOI: https://doi.org/10.3390/met11020321 DOI: https://doi.org/10.3390/met11020321
J. Roman, S. Mišković. “Process Intensification of Metal Solvent Extraction Studies using a Miniaturized Solvent Extraction Plant”. ChemRxiv, 2023, pp. 26 DOI:https://doi.org/10.26434/chemrxiv-2023-nc9q1 DOI: https://doi.org/10.26434/chemrxiv-2023-nc9q1
P. Yudaev, E. Chistyakov. “Chelating Extractants for Metals”. Metals, vol. 12, no. 8, 2022. DOI: https://doi.org/10.3390/met12081275 DOI: https://doi.org/10.3390/met12081275
M. T. Collins, E. J. McConaghy, R. F. Stauffer, G. J. Desroches and B. D. Krysa, “Starting up the Sherritt Zinc Pressure Leach Process at Hudson Bay”, JOM, vol. 46, no. 4, pp. 51–58, 1994. DOI: https://doi:10.1007/bf03220675. DOI: https://doi.org/10.1007/BF03220675
E. Weidenhammer. Developments in Canadian Hydrometallurgy since 1950. Canada, Canada Science & Technology, 2021.
M. E. Chalkley, E. Ozberk and W. D. Vardill, “The treatment of bulk concentrates by the sherritt zinc pressure leach process”, Minerals Eng., vol. 6, no. 8-10, pp. 937–948, Aug. 1993. DOI: https://doi:10.1016/0892-6875(93)90066-v. DOI: https://doi.org/10.1016/0892-6875(93)90066-V
K.R Buban, M.J. Collins, and LM. Masters, "Iron control in zinc pressure leach processes" JOM, vol.51, no. 12, pp. 23-25, 1999. DOI: https://doi.org/10.1007/s11837-999-0166-8
“Zinc Applications Archives | ZINC. International Zinc Association”. Home | ZINC. International Zinc Association. Accessed: Jun. 2023. [Online]. Available: https://www.zinc.org/category/learning-annex/zinc-applications/
“Zinc: reservas mundiales por países 2022 | Statista”. Statista. Accessed: May 8, 2023. [Online]. Available: https://es.statista.com/estadisticas/635671/reservas-mundiales-de-zinc-por-paises/
A. Akcil, Z. Sun y S. Panda, “COVID-19 disruptions to tech-metals supply are a wake-up call”, Nature, vol. 587, n.º 7834, pp. 365–367, Nov. 2020. DOI: https://doi:10.1038/d41586-020-03190-8. DOI: https://doi.org/10.1038/d41586-020-03190-8
International Zinc Association, “Zinc Recycling Report 2050: Supply and Demand”. Accessed: May 14, 2023. [Online]. Available: www.zinc.org.
A. Siegmund, S. Alam, J. Grogan, U. Kerney and E. Shibata. Pb-9th International Symposium on Lead and Zinc Processing. The Minerals, Metals & Materials Series. Springer, 2020. DOI: https://doi:10.1007/978-3-030-37070-1 DOI: https://doi.org/10.1007/978-3-030-37070-1
C.M. Backman, “Global Supply and Demand of Metals in the Future”, J. Toxicol. Environmental Health, Part A, vol. 71, no. 18, pp. 1244–1253, Aug. 2008. DOI: https://doi:10.1080/15287390802209582. DOI: https://doi.org/10.1080/15287390802209582
L. Rostek, E. Pirard and A. Loibl, “The future availability of zinc: Potential contributions from recycling and necessary ones from mining”, Resources, Conservation & Recycling Advances, p. 200166, Jun. 2023. DOI: https://doi:10.1016/j.rcradv.2023.200166. DOI: https://doi.org/10.1016/j.rcradv.2023.200166
L. Rostek, L. Tercero Espinoza, D. Goldmann and A. Loibl, “A dynamic material flow analysis of the global anthropogenic zinc cycle: Providing a quantitative basis for circularity discussions”, Resources, Conservation Recycling, vol. 180, p. 106154, May 2022. DOI: https://doi:10.1016/j.resconrec.2022.106154. DOI: https://doi.org/10.1016/j.resconrec.2022.106154
“Mineral commodity summaries 2023”, US Geological Survey, 2023. DOI: https://doi:10.3133/mcs2023. DOI: https://doi.org/10.3133/mcs2023
K. Binnemans y P. T. Jones, “The Twelve Principles of Circular Hydrometallurgy”, J. Sustain. Metall., December 2022. DOI: https://doi:10.1007/s40831-022-00636-3. DOI: https://doi.org/10.1007/s40831-022-00636-3
L. H. Xavier, M. Ottoni y L. P. P. Abreu, “A comprehensive review of urban mining and the value recovery from e-waste materials”, Resources, Conservation Recycling, vol. 190, p. 106840, Mar. 2023. DOI: https://doi:10.1016/j.resconrec.2022.106840. DOI: https://doi.org/10.1016/j.resconrec.2022.106840
A. Arguillarena, M. Margallo,A. Arruti-Fernández, J. Pinedo, P. Gómez, I. Ortiz and A. Urtiaga, “Circular economy in hot-dip galvanizing with zinc and iron recovery from spent pickling acids”, RSC Advances, vol. 13, no. 10, pp. 6481–6489, 2023. DOI: https://doi:10.1039/d2ra08195d. DOI: https://doi.org/10.1039/D2RA08195D
Report on the Environmental Benefits of Recycling Bureau of International Recycling, 2016 https://www.bir.org/images/uploads/publications/BIR-CO2-report-2016-FIN-WEB.pdf
E. Rudnik, “Investigation of industrial waste materials for hydrometallurgical recovery of zinc”, Minerals Eng., vol. 139, p. 105871, Aug. 2019. DOI: https://doi:10.1016/j.mineng.2019.105871. DOI: https://doi.org/10.1016/j.mineng.2019.105871
M. Kaya, S. Hussaini, S. Kursunoğlu. Critical review on secondary zinc resources and their recycling technologies. Hydrometallurgy, 2020.105362. DOI: https://doi:10.1016/j.hydromet.2020.10536 DOI: https://doi.org/10.1016/j.hydromet.2020.105362
Z. Sun, Y. Xiao, H. Agterhuis, J. Sietsma, Y. Yang. “Recycling of metals from urban mines – a strategic evaluation”. Journal of Cleaner Production, vol. 112, part 4, pp. 2977-2987, 2015. DOI: https://doi:10.1016/j.jclepro.2015.10.116. DOI: https://doi.org/10.1016/j.jclepro.2015.10.116
C. Hageluken. “Recycling of electronic scrap at Umicore’s integrated metals smelter and refinery”. Proceedings of European Metallurgical Conference, vol. 59, pp. 307-323, 2005.
R. Rajesh, D. Kanakadhurga and N. Prabaharan, “Electronic waste: A critical assessment on the unimaginable growing pollutant, legislations and environmental impacts”, Environmental Challenges, vol. 7, p. 100507, Apr. 2022. DOI: https://doi:10.1016/j.envc.2022.100507. DOI: https://doi.org/10.1016/j.envc.2022.100507
S. M. Sadeghi, J. Jesus y H. M. V. M. Soares, “A critical updated review of the hydrometallurgical routes for recycling zinc and manganese from spent zinc-based batteries”, Waste Manage, vol. 113, pp. 342–350, Jul. 2020. DOI: https://doi:10.1016/j.wasman.2020.05.049. DOI: https://doi.org/10.1016/j.wasman.2020.05.049
N. Štrbac, I. Mihajlović, V. Andrić, Ž. Živković y A. Rosić, “Kinetic investigations of two processes for zinc recovery from zinc plant residue”, Can. Metallurgical Quart., vol. 50, no. 1, pp. 28–36, Jan. 2011. DOI: https://doi:10.1179/000844311x552287. DOI: https://doi.org/10.1179/000844311X552287
V. Forti, C. P. G. Balde, R. Kuehr y B. Garam, The Global E-waste Monitor 2020: Quantities, flows and the circular economy potential. Bonn/Geneva/Rotterdam: United Nations University (UNU)/United Nations Institute for Training and Research (UNITAR) – co-hosted SCYCLE Programme, International Telecommunication Union (ITU) & International Solid Waste Association (ISWA), 2020.
International Lead and Zinc Study Group, “The world zinc factbook 2024”. [Online]. Available: https://www.ilzsg.org/wpcontent/uploads/SitePDFs/The%20World%20Zinc%20Factbook%202024.pdf
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2025 EPISTEMUS

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
La revista adquiere los derechos patrimoniales de los artículos sólo para difusión sin ningún fin de lucro, sin menoscabo de los propios derechos de autoría.
Los autores son los legítimos titulares de los derechos de propiedad intelectual de sus respectivos artículos, y en tal calidad, al enviar sus textos expresan su deseo de colaborar con la Revista Epistemus, editada semestralmente por la Universidad de Sonora.
Por lo anterior, de manera libre, voluntaria y a título gratuito, una vez aceptado el artículo para su publicación, ceden sus derechos a la Universidad de Sonora para que la Universidad de Sonora edite, publique, distribuya y ponga a disposición a través de intranets, internet o CD dicha obra, sin limitación alguna de forma o tiempo, siempre y cuando sea sin fines de lucro y con la obligación expresa de respetar y mencionar el crédito que corresponde a los autores en cualquier utilización que se haga del mismo.
Queda entendido que esta autorización no es una cesión o transmisión de alguno de sus derechos patrimoniales en favor de la mencionada institución. La UniSon le garantiza el derecho de reproducir la contribución por cualquier medio en el cual usted sea el autor, sujeto a que se otorgue el crédito correspondiente a la publicación original de la contribución en Epistemus.
Salvo indicación contraria, todos los contenidos de la edición electrónica se distribuyen bajo una licencia de uso y Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0) Puede consultar desde aquí la versión informativa y el texto legal de la licencia. Esta circunstancia ha de hacerse constar expresamente de esta forma cuando sea necesario.